In: Metal Ions in Biological Systems, Vol. 26: Compendium on magnesium and its role in biology, nutrition and physiology. H. Sigel and A. Sigel eds. Marcel Dekker Inc. New York-Basel 1990: 744 p.2, 4, 243-247
Magnesium and Its Relationship to Oncology
Jean Durlach
64, Rue de Longchamp, F-92200 Neuilly, France
Michel Bara and Andrée Guiet-Bara
Université P. et M. Curie, F-75006 Paris, France
1. INTRODUCTION
2. INTERACTION BETWEEN CARCINOGENESIS AND MAGNESIUM STATUS
2.1 Magnesium Disturbances by Carcinogenesis
2.2 Effect on Carcinogenesis Induced by Disturbances of Mg Status
2.2 Effect on Carcinogenesis Induced by Disturbances of Mg Status
3. CELLULAR INTERACTION BETWWEN CANCER AND MAGNESIUM STATUS
3.1. Uptake of Magnesium in Normal and Tumor Cells
3.2. Plasma Membrane, Magnesium, and Cancer
3.3. Postmembrane and Prenucleus Magnesium and Cancer Interactions
3.4. Nucleus, Magnesium, and Cancer
3.2. Plasma Membrane, Magnesium, and Cancer
3.3. Postmembrane and Prenucleus Magnesium and Cancer Interactions
3.4. Nucleus, Magnesium, and Cancer
4. SYSTEMIC MECHANISMS OF THE RELATION BETWEEN MAGNESIUM AND CANCER
4.1 Metabolic Basis of Interaction Between Magnesium and Cancer
4.2. Magnesium, Cancer, and Immunity
4.3. Magnesium, Cancer, and Cell Growth
4.2. Magnesium, Cancer, and Immunity
4.3. Magnesium, Cancer, and Cell Growth
5. NEW PERSONAL DATA
6. GENERAL CONCLUSIONS
6.1. General Scheme of Relationship Between Magnesium Status and Cancer
6.2. Magnesium and Cancer Research
6.3. Magnesium and Cancer Treatment
6.2. Magnesium and Cancer Research
6.3. Magnesium and Cancer Treatment
ACKNOWLEDGMENT
REFERENCES
1. INTRODUCTION
Relationships between magnesium and cancer are complex: both Mg load and Mg deficit may produce either carcinogenic or anticarcinogenic effects. Carcinogenesis modifies the Mg status inducing Mg distribution disturbances which may frequently associate a tumor Mg load with Mg depletion in nonneoplastic tissues1-3.
The aim of this chapter is first to review the consequences of the cancerous state on Mg metabolism and those of the disturbances of Mg status on carcinogenesis, to analyze the cellular and systemic bases of these relations, and to appreciate their theoretical and practical implications. We will then present some new personal data on the membranous relationship between magnesium and various anticancer or carcinogenic agents. We will attempt as a conclusion to show how these data may bring about new promising developments in cancer research as well as in Mg and anticancer treatment.
2. INTERACTION BETWEEN CARCINOGENESIS AND MAGNESIUM STATUS
2.1. Magnesium Disturbances by Carcinogenesis
During chemical carcinogenesis it is possible to observe Mg cellular deficit in preneoplastic and neoplastic states. Mg deficit causes structural and functional alterations of the plasma membrane with a decrease of the intracellular cations Mg and K and an increase of the extracellular cations Ca and Na in neoplastic cells. These drastic changes are grossly similar to the cellular alterations observed in magnesium deficiency1-4. One of the principles for the prophylactic use of Mg in oncology is to avoid the facilitation of the effect of a carcinogenic agent by the induction of cellular disturbances of this type1-4. In fact, the disorders in Mg distribution in carcinogenesis are far more complex than those observed in simple Mg deficiency. During the neoplastic state, Mg bound in intracellular structures decreases for the most part while its concentration in the cytosol1-4 -- and sometimes in mitochondria5 -- increases. This type of disturbance in Mg distribution, unobserved in simple Mg deficiency, indicates an alteration of the intracellular Mg regulation, i.e., a type of Mg depletion even at an early stage of carcinogenesis1-3. At a later stage, disturbances in Mg distribution are even more complex. In man, at a later cancerous stage, the disturbance in Mg distribution associates an increased Mg level in the tumor1-4,6-8 and in blood cells including erythrocytes and lymphocytes1-4,6, with some stigmata of Mg depletion which differ according to the clinical type and treatment1-4,6-13. For example, the Mg level of breast cyst fluids does not constitute a risk indicator for breast cancer14. A high concordance exists between high Mg/Ca ratio levels in meningiomas and the absence of microscopic calcifications, which may testify to the inhibitory potency of Mg against mineralization15. Mg depletion appears more frequently in patients with solid tumor malignancy than with hemopathic malignancy16. Radiation enteritis increases fecal Mg loss3,17 and cancer cachexia urinary Mg loss18 in particular. Mg depletion does not occur equally in all tissues. Experimental and clinical data show that usually the adverse effect of Mg depletion in nonneoplastic tissue are reversible1-3,17,19.
It seems, therefore, that an established cancer induces Mg disturbances which cause Mg load in tumoral tissue, possibly due to Mg mobilization through blood cells, with Mg depletion in nonneoplastic tissue1-4,6.
2.2. Effect on Carcinogenesis Induced by Disturbances of Mg Status
Mg deficiency may produce either tumorogenic or anticarcinogenic effects. In reverse, Mg acts either as an anticancer or a carcinogenic agent.
2.2.1. Tumorogenesis Due to Mg Deficit and Anticancer Effects of Mg
In the rat only, a prolonged Mg deficiency induces the development in a minority of animals of either benign lesions (tumor-like connective proliferation in the intestine1,2) or periosteal desmoid tumor formation20, or malignant defects thymic lymphoma and myeloid leukemia1-4, 6,11). There is no correlation between the development of benign and malignant proliferations. Genetic factors are prominent: Mg deficiency does not induce oncogenicity in any species other than the rat, or in any rat except in a minority of rats of the same particular strains1-3,21. The anticarcinogenic properties of Mg observed on this particular model provide only poor support to the general notion of the anticarcinogenic properties of Mg. In several models of benign or premalignant tumorogenesis, Mg inhibits the development of the defect: on established benign hyperplastic lesions induced by topical application of DMBA (7,12-dimethylbenzanthracene) in hamster cheek pouch2 and on two premalignant esophageal tumors induced in rats either by MBN (N-nitro-somethylbenzylamine)22 or mainly by a corn-fed diet23 of the same type as that of the Zulu population with a high risk of esophageal cancer24. It is very important to emphasize that the preventive effect of Mg exists only at the early stage of tumorogenesis. This interesting experimental notion inspires study of the prophylactic anticarcinogenic power of Mg, particularly in populations where a high risk of tumorogenesis and a marginal or insufficient intake of Mg coexist25, i.e., in a population with colorectal polyps26. Usually, Mg supplement alone does not favorably influence the development of established cancer1-4,6 with the only exception being an inhibition of the development of MAM (methylazoxymethanol acetate)-induced large-bowel carcinogenesis in rat27 with high cathartic doses of magnesia.
2.2.2. Inhibition of Tumorogenesis Due to Mg Deficit and Carcinogenic Effects of Mg
It has been well established for almost 25 years that Mg deficiency antagonizes tumor implantation and inhibits growth of induced or spontaneous tumors in the rat. In reverse, Mg intake stimulates tumor development in the rat1-4,6,28-31. Concurrently, Mg deficiency--associated with K deficiency--induced by diet and hemodialysis has been used in man as an anticancer treatment to slow the progression of inoperable cancer. With questionable therapeutic efficiency, such attempts carry the risk of obtaining results at the price of distressing secondary effects1-3,6.
These data on the effect of Mg deficiency in several neoplastic states show that Mg seems to be anticarcinogenic in the case of early premalignant states and of some hemolymphoreticular malignancies, and most often to be carcinogenic in the case of solid tumors1-4,6.
3. CELLULAR INTERACTION BETWEEN CANCER AND MAGNESIUM STATUS
The metabolism of proliferative and normal cells is markedly different and the links between cancer and magnesium status may rely on cellular and subcellular backgrounds.
3.1. Uptake of Magnesium in Normal and Tumor Cells
The concentration of intracellular free Mg is mainly regulated by adaptations of Mg efflux. When the intracellular free Mg is Mg-depleted, nongrowing cells do not take up Mg. The uptake of cellular Mg is possible when the normal cells are moderately depleted and are growing. But tumor cells, e.g., Ehrlich or Yoshida ascites tumor cells, or tumorogenic pancreatic β cells or thymocytes rapidly reaccumulate Mg. This Mg influx in tumor cells is inhibited sometimes by furosemide33, sometimes by amiloride35. Newly transported Mg2+ exchanges extensively with cytoplasmic Mg suggesting that compartmentation of Mg2+ may be dependent on proliferative status. Any alteration of Mg distribution in the cancer cell may play an important role in the neoplastic development at the membrane, cytosol, or nucleus levels1-3,32-35.
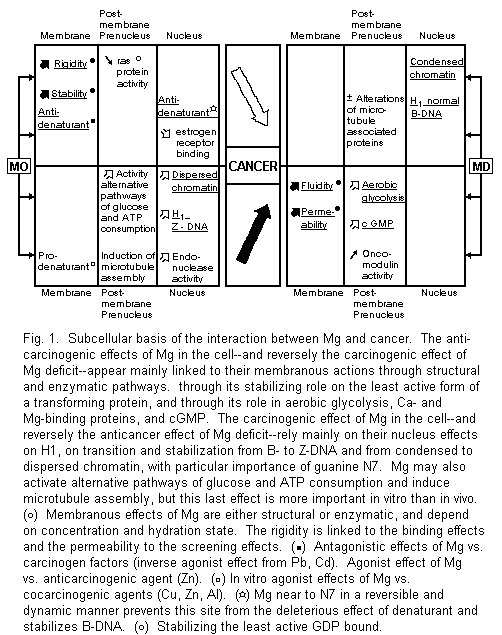
3.2. Plasma Membrane, Magnesium, and Cancer
Accumulated evidence suggests that there is a common feature underlying the diversity of tumors, i.e., the alterations of their biomembrane system. It is very interesting to point out the similarities between the plasma membrane impairment due to malignancy and magnesium deficiency. Both increase fluidity and permeability1-4,6,36. The stabilizing properties of Mg are due first to its structural role. Mg is necessary for the insertion of proteins into membranes and for the formation of the phospholipids with parallel electrostatic polarizing effects. The rigidifying action of Mg is tied to the decrease of the motional freedom of phospholipids due to their binding effect with Mg. Conformational changes induced by Mg often depend on Mg-dependent transitions. For example, the Mg ion exerts a central role in the regulation of membrane receptors, promoting interconversion from monomer to dimer forms1-3,36-38. However, this molecular basis for membrane Mg actions does not exclude enzymatic pathways. Neoplastic transformation of multiple cell lines reduces the activity of numerous membrane Mg-dependent enzymes, i.e., Na+, K+-ATPase and Ca2+, Mg2+-ATPase 1-3, 39, 40. All these data are relevant to the anticarcinogenic properties of Mg.
Our recent in vitro studies on the human amniotic membrane model showed that Mg -- at physiological dose range -induces a biphasic effect on the maternal and fetal side. Ionic fluxes first decrease, then increase, but without alterations of the flux ratio. This screening effect followed by a binding effect seems closely linked with hydrated and dehydrated forms of Mg2+ ions41,42. A partial approach of these observations may involve hazardous conclusions. Several mechanisms of the anticancer action of Mg may be suggested. For example, since a part of the screening effect of Mg2+ may provene from stimulation of gap cellular communication, it may antagonize the inhibition of this process considered as a promotion phase of carcinogenesis43. Since all carcinogenic and cocarcinogenic agents induce a decrease of ionic fluxes with alteration of the flux ratio, the reverse actions of the binding effect of Mg2+ might act as a general antidote of these pollutants. Our earlier and recent data have well established the erroneous character of these hypotheses. The action of Mg is biphasic and should not be restricted to its binding effect. Mg may sometimes be a competitive or noncompetitive antagonist vs. carcinogenic pollutants, but is may also be inactive on them and sometimes even act as a specific or nonspecific agonist1-3,45. These discrepancies may be partly accounted for by dissimilarities in hydrated ionic radii, but the importance of the selected models and of the experimental conditions should not be overlooked. For instance, various cations such as Mg2+, Ca2+, Zn2+, and Pb2+ may either compete with or replace each other1-3,28,44-46which may have anticancer or carcinogenic implications.
3.3. Postmembrane and Prenucleus Magnesium and Cancer Interactions
These postmembranous interactions can either be anticarcinogenic or carcinogenic.
3.3.1. Postmembrane and Prenucleus Anticarcinogenic Actions of Mg and Carcinogenic Effects of Mg Deficit
In cancer cells, the Ca/Mg ratio-dependent aerobic glycolysis enhancement is mediated by phosphofructokinase activation. This is the key enzyme in the regulation of glycolysis. Ca load and Mg deficiency might be responsible for its activation1,2,4.
A basic effect caused by Mg deficiency is an increased cGMP level and cGMP seems capable of stimulating normal and malignant cell growth1-3.
Mg stabilizes the least active GDP-bound form of the transforming protein induced by ras genes47.
Since oncomodulin, a tumor bivalent-cation binding protein has calmodulin-like activity, the possibility of a Mg antagonism can also be considered there1-3, but reversely Mg may also stimulate the effects of calmodulin and Ca on growth regulations acting as a carcinogenic agent48.
3.3.2. Postmembrane and Prenucleus Carcinogenic Actions of Mg and Anticarcinogenic Effects of Mg Deficit
Mg2+ is able to modify the energy metabolism of Ehrlich ascites tumor cells and to activate alternative pathways responsible for glucose and ATP consumption. It decreases lactate and pyruvate production, and increases glucose consumption with a rapid drop of ATP levels associated with an unexpected parallel drop of the other two nucleotides, ADP and AMP49.
Mg2+ can usually replace several polyamines and putrescine in particular in many reactions. But it is not the case that the tumorogenic property of putrescine reestablishes the growth rate of Ehrlich ascites tumor cells inhibited by α-difluoromethylornithine, an enzyme-activated irreversible inhibitor of ornithine decarboxylase50.
In several in vitro models, Mg2+ is critical for induction of microtubule protein assembly including tubulin and microtubule-associated proteins. It stimulated its polymerization and then permits both elongation and nucleation reactions, facilitating mitosis, cell division, and growth51,52. Nevertheless, in the Mg-deficient rat its importance in vivo seems abated, with only minor alterations in the associated protein and without any changes in the polymerization rate53.
In the above-mentioned biochemical reactions, Mg usually acts as a carcinogenic agent.
The most important recent advances concerning the relationship between Mg and cancer rest on the findings concerning the relation between Mg and the cell nucleus, which are most often biphasic.
3.4.1. Mg, Chromatin, and Histones
The presence of increasing Mg2+ in intact cell nuclei affects their spatial organization. With a laser flow microfluorimeter coupled to a phase contrast analyzer, it is possible to identify a shrinking process induced by Mg2+ in the range 0.4-2.5 mM, which reaches a plateau in the range 5-20 mM and is followed by a swelling process for the highest concentration in the range 30-60 mM. It is tempting to speculate that the shrinking-swelling phenomenon has a molecular correspondence at the genome level. The same Mg ranges are shown to affect the intercalation of the flurochrome acridine orange into chromatin. The process of acridine orange intercalation can be considered as a stimulation of the accessibility of the genome to biological molecules. During the shrinking phenomenon a high level of chromatin condensation is preserved and during the swelling phenomenon chromatin is dispersed, leading to an increased accessibility to the cell genome and to the higher activation of cell functional processes. Among the main cellular cations, Mg2+ is the one with the most influence on the chromatin structure. It is known that larger amounts of Na+ ions are needed to cause the same effect1,2,54.
Histones, and histone H1 in particular, stabilize the structure of chromatin against changes in ionic strength, i.e., against high concentrations of Mg. This regulation may fail when the concentration of Mg increases. The histones exhibit an increased content of elongated left-handed helices of the poly-L-proline II type. However, Mg deficiency in the rat at an early stage alters the histone H1. But this disorder is compensated for in the course of the experimental deficiency, presumably by a subtle subcellular regulation due to a release of Mg from other subcellular sites into chromatin. It seems to be equally important to maintain normal levels of Mg and histone H1 in chromatin in order to benefit form their critical role in higher order structures. The Mg2+ content of histone fractions, namely, H1, of ascite hepatome 22A tumor cells is higher than in normal liver cells in the mouse. Variations in chromatin compactness is an early effect induced by chemical carcinogens in vivo. Nickel, a potent carcinogenic metal, induces its chromosomal damaging activity through a preferential linking in magnesium-insoluble regions of fractionated chromatin which include histone H1. These Mg alterations may have an etiological role in higher order structure disturbances1-3, 55-59.
Further studies of the mechanisms controlling cellular and subcellular Mg distribution3 and histone interactions55 will open new paths to cancer research.
3.4.2. Mg, Cancer, and Nucleic Acids
In the nucleus, DNA constitutes one of the selective targets of Mg. For example, a flow cytofluorographic technique has been able to accessin vivo increased DNA synthesis induced by parenteral Mg injections in placenta cells of pregnant women60. Numerous in vitro studies indicate that hexahydrated Mg ion binds directly to the guanine N7 and O6 atoms and to the negative oxygens of the phosphate through the water molecules of hydration. The Mg atom is found to be near the N7 site in a reversible and dynamic manner. It might prevent this site from being attacked by denaturants; this may be one reason why a normal Mg status is preventive against cancer1-3,61,62. Mg2+, as well as several polyamines63, can generate the transition from left-handed conformation of DNA (B-DNA) to its right-handed conformation (Z-DNA). The synthetic polynucleotide poly (dG-M5dC) shows a right-handed form in all samples when the ratio Mg/nucleotides is > 190/1000 and a left-handed conformation when the same ratio is < 45/100064. When an increased concentration of Mg induces and stabilizes the transition form B-DNA to Z-DNA, it may stimulate carcinogenesis. Similarly, some well-known carcinogens such as N2-acetylaminofluorene and Ni carcinogenic compounds may stimulate carcinogenesis1-3,57-59. On the contrary, some anticancer agents like cisplatin or SOAZ may act as N7-site Mg-excess antagonists or modulators by stabilizing the B-DNA geometry1-3,61,62. Although the effects of Mg on DNA guanine are well known, its effects on other DNA targets, such as, for example, adenine (where N3 seems linked to carcinogenesis) or on RNA deserve consideration1-3,62.
Thus the relationship between Mg and nucleic acids confirms the importance of the regulation of subcellular Mg distribution1-3, particularly on the N7 site of guanine: when normal, it protects against carcinogenesis; with abnormally high levels, it generates the Z conformation of DNA which is correlated with carcinogenicity.
3.4.3. Mg, Cancer, and Other Nuclear Fractions
Several endonucleases stimulated by Mg (and Ca) seem to be involved in cell proliferation and can be useful enzymatic probes for detecting chromatin changes65,66. In experimental and human mammary tumors, Mg and nuclear estrogen receptor are linked. Mg2+ ions inhibit the nuclear binding of the receptor67,68. These data are scarce but seem promising for new directions of research.
4. SYSTEMIC MECHANISMS OF THE RELATION BETWEEN MAGNESIUM AND CANCER
Mg may interfere with malignancy through its multiple roles in all the systems of the organism but mainly through its metabolic, immunological, and growth-stimulating effects.
4.1. Metabolic Basis of Interaction Between Mg and Cancer
These metabolic interactions are anticarcinogenic and carcinogenic (Fig. 2).
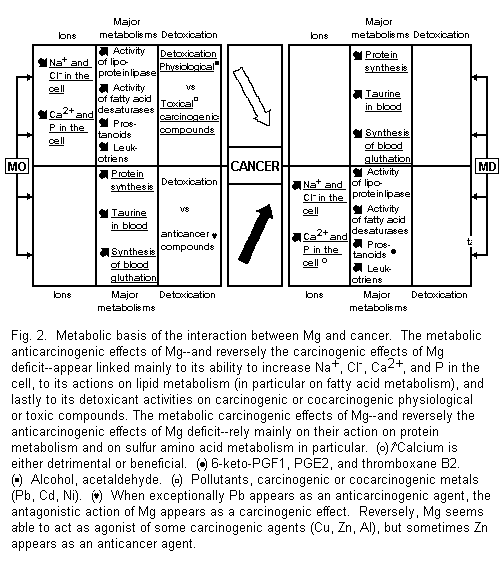
4.1.1. Metabolic Anticarcinogenic Actions of Mg and Carcinogenic Effects of Mg Deficit
Among the drastic changes in cancer and magnesium-deficient cells an increase of Na (and Cl) and of Ca (and P) involve grossly similar features. Mg load may tend toward a reduction of these ionic cellular increased concentrations1-4,38. High levels of Na+ and Cl- are associated with rapid cell reproduction and transformation. The amiloride caused decrease of these ions is correlated with a decreased cell proliferation69-72. The significance of calcifications in tumor cells is more dubious, most often detrimental, but also protective1-4,15,38,73.
Mg deficiency produces several variations in lipid metabolism which may participate in carcinogenic processes, e.g., reduced activity of the lipoprotein-lipase system and disturbances of fatty acid metabolism, involving inhibition of several steps of desaturation, increased synthesis of prostanoids (i.e., in the Mg-deficient rats significantly higher concentrations of 6-keto-PGF-1α, PGE2, and thromboxane B2) and leukotriene B41-3,38,74-77. It is interesting to stress the similar striking elevation of thromboxane B2 in thymocytes from magnesium-deficient rats, which are capable of developing thymic lymphoma and myeloid leukemia, and from AKR mice (8-12 months old) developing leukemia75,78. Although magnesium deficiency in men and rat causes a magnesium-curable hyperaggregation of platelets3,74, hyperaggregation of platelets due to tumor cells does not appear to be Mg-dependent79.
The metabolic anticancer properties of Mg may come from its antagonism against carcinogenic or cocarcinogenic agents. This antidenaturant power concerns physiological or toxic compounds1-3,29,30,45,56-59,61,62,84,85. Both our earlier and our recent data in vitro on human amnion in particular and in vivo in mice have shown such antagonistic properties against alcohol, and acetaldehyde, Cd and Pb at different competitive sites; and Ni by noncompetitive action1-3,45,80-82. In a few cases where Zn acts as an anticarcinogenic agent46,86,87, its activation by Mg45 appears as an anticancer effect.
All these metabolic actions of Mg are relevant to its carcinogenic effects.
4.1.2. Metabolic Carcinogenic Actions of Mg and Anticarcinogenic Effects of Mg Deficit
Among the numerous effects of Mg on protein metabolism, which plays a prominent part in mechanisms of cell growth, its role in sulfur amino acid metabolism deserves particular attention. The inhibition of tumor growth due to Mg deficiency in the rat may depend on mobilization of taurine and inhibition of the biosynthesis of blood glutathione1-3,37, which evidently depends on sulfur amino acid intake and metabolism83.
Our recent in vitro observations on human amnion of an activation of three cocarcinogenic agents on common sites for Al and Cu, and without specificity for Zn, may indicate the possibility of an agonistic action of Mg against some denaturants. In exceptional cases where Pb appears as an anticarcinogenic agent28, the antagonistic action of Mg appears as a carcinogenic effect. These metabolic actions of Mg agree with some carcinogenic powers of this ion.
4.2. Magnesium, Cancer, and Immunity
Recent advances concerning the systemic interaction between Mg and cancer rely on the close and complex links between Mg and the immune system1-3,88-90. They are anticarcinogenic and carcinogenic (Fig. 3).
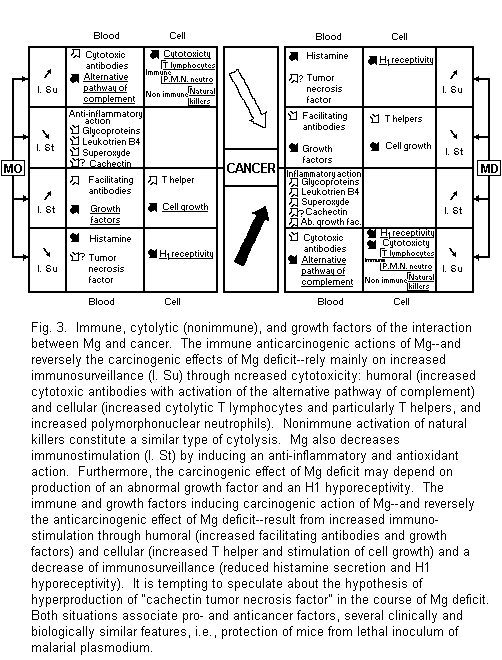
4.2.1. Immune Anticarcinogenic Action of Mg and Carcinogenic Effect of Mg Deficit
The anticarcinogenic action of Mg rests of Mg stimulation of cancer immunosurveillance and inhibition of immunofacilitation. Reverse mechanisms concern the carcinogenic effects of Mg deficit. The paths are either humoral or cellular.
Mg stimulates synthesis of cytotoxic antibodies. Simultaneously its activation of an alternative pathway of complement increases their inhibiting effect on tumor growth1-3,88-90,91-93. Mg-dependent immune tumor cytolysis is mediated by blood monocytes, mainly by T lymphocytes, and by polymorphonuclear neutrophils with a mechanism of tumor cell injury due to activated oxygen1-3,88-90,94-95. Nonimmune antitumor surveillance mediated by natural killers also requires Mg2+ for target cell lysis1-3,84.
Study of immune disturbances of Mg deficiency shows the reverse mechanisms of cancer immunosurveillance inhibition and allows better identification of the type of T cytolytic lymphocyte concerned. The percentage of total T lymphocyte and that of T lymphocyte with helper function are reduced whereas the percentage of T lymphocyte with suppressor function is enhanced21. Futhermore, Mg deficit induces an oncogenic inflammatory reaction with an increase of phlogistic glycoprotein, leukotriene B4, and superoxide anion3,77,88-90,96,97. Lastly, the same low responsiveness of histamine releasers is similarly noted in spleen cells of rat with experimental Mg deficiency and in rat basophilic leukemia cells98. These immune data and those concerning nonimmune natural killers are consistent with some anticancer properties of Mg.
4.2.2. Immune Carcinogenic Actions of Mg and Anticarcinogenic Action of Mg Deficit
Among the major features in cancer immunosurveillance and in experimental and clinical magnesium deficiency, hyperhistaminemia and histamine hyperreceptivity appear to be similarly important. Part of the anticarcinogenic action of Mg deficit may be compared with clinical improvement observed in some advanced cancer diseases after a treatment combining histamine and H2 antihistaminics. This mechanism does not exclude a hypothetical increased level of the tumor necrosis factor in blood nor an inhibition of the immunofacilitation due to the immune carcinogenic actions of Mg. Mg may stimulate synthesis of antibodies and of T lymphocytes which induce tumor growth1-3,88-90,98-100.
These data are relevant to some antitumor effects of Mg deficit. It seems interesting to emphasize a number of similarities between the effects of hyperproduction of cachectin and those observed in a Mg-deficient state. Both situations associate pro- and anticancer factors. Both reduce activity of lipoprotein lipase and blood pressure, may lead to disseminated intravascular coagulation, induce an imflammatory reaction with an increased level of leukotrienes, similarly alter membrane permeability, activate polymorphonuclear leukocytes, and lastly protect mice from lethal inoculum of malarial plasmodium1-3, 101, 102. It is therefore tempting to speculate regarding the hypothesis of a hypersecretion of "cachectin tumor necrosis factor" during magnesium deficit.
Immunostimulation and immunosuppression may be either useful or noxious in cancer treatment. The beneficial or detrimental effects of Mg load and Mg deficit agree with this notion. But, in fact, hemolymphoreticular malignancies are usually under immunosurveillance: in such cases the Mg stimulating effects are useful1-3,8. However, most often solid tumors are immunostimulated. It is therefore advisable to be careful with all types of immunostimulation and particularly with Mg treatment. This rule admits some exceptions, e.g., the control of a badly tolerated Mg deficit, especially when it is induced by a side effect of an efficient cytolytic treatment like cis-Pt 1-3.
4.3. Magnesium, Cancer, and Cell Growth
Indirectly through mediation of growth factors and directly through its effects on the cell, Mg stimulates normal and proliferative cell growth. This action constitutes one of the main mechanisms of its carcinogenic property.
4.3.1. Direct Effect of Mg on Cell Growth
Magnesium is an essential factor of cell growth. Free Mg2+ permits tubulin polymerization and subsequent spindle formation. It triggers chromosome condensation and eventually spindle breakdown, enabling nuclear and cell division to proceed. In tumor cells, uptake of Mg and cell growth are coordinated processes. Mg plays a central role in the proliferative response1-3,38,47,103-105. Its effects are more deleterious because the Mg requirement in transformed cells is less than in normal cells1-3,106.
4.3.2. Magnesium, Cancer, and Growth Factors
Growth factors in oncology may induce two noxious effects: either through their mitotic actions by stimulating cell proliferation or through neoangiogenesis by acting as angiogenic factors107. Mg mediates and promotes the effects of several serum-derived hormone-like growth factors, epidermal growth factors, and nerve growth factors1-3,106-110.
Unexpectedly, Mg deficiency-induced malignant T-cell lymphomas produce a particular protein growth factor with a molecular weight of 20'000-25'00021. These stimulating effects of Mg on growth may participate in some of its carcinogenic properties, but paradoxically a particular growth factor may also intervene in some carcinogenic properties of Mg deficiency-induced lymphoma.
5. NEW PERSONAL DATA
Previous model studies1,2,45 on the human amniotic membrane, a leaky, asymmetric, and nonexcitable membrane, indicated that Mg is not an antagonist of all carcinogenic metals. These data have shown the essential role of the membrane in the case of a competitive inhibition between Mg and metals, but also another action level, e.g., the nucleus and nucleic acids, in the case of a noncompetitive inhibition and an absence of action.
The present data determine the relationship between Mg and anticancer agents, and between Mg and alcohol.
5.1. Magnesium and Anticancer Agents
5.1.1. Magnesium and Anticancer Metals or Metalloids
In recent studies1,2 the relationship between Mg and two anticancer metals, i.e., gallium (GA) and cisplatin (Cis-Pt), has been observed. Ga and Cis-P5 decrease the ionic permeability through the human amnion, estimated by the measurement of the total ionic conductance (Gt) on the two sides (maternal and fetal), except that Cis-Pt already does this at low concentration on the fetal side. There is a noncompetitive inhibition between Mg and Ga, i.e., Mg and Ga are fixed on different sites on the surface membrane. Moreover, there is no antagonism between Mg and Cis-Pt.
Selenium, a metalloid, may be considered with regard to its anticancer properties. The Se-mediated anticarcinogenesis seems to be due to an action on glutathione, and this action is identical to the effects observed in the case of magnesium deficiency1. In the human amnion, Se decreases Gt on the maternal side, but has no effect on the fetal side (Fig. 4). There is a noncompetitive antagonism between Mg and Se on the maternal side (Fig. 5); Mg and Se are fixed on different sites on the surface membrane.
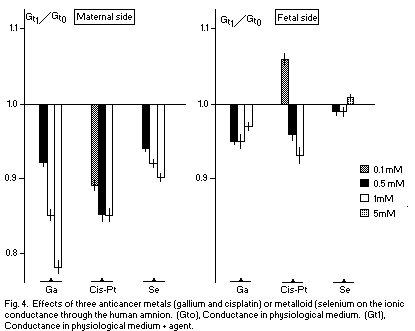
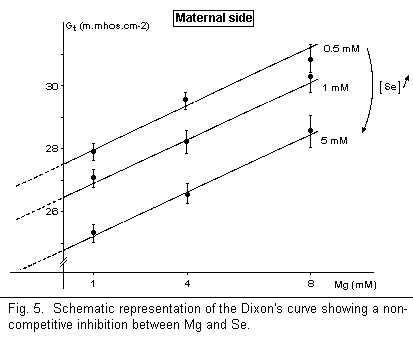
The reduction of the membrane conductance Gt, a deleterious effect, observed in these data indicates that the anticarcinogenic effects of Ga, Cis-Pt, and Se are located on targets other than the plasmatic membrane. The noncompetitive antagonism (Mg-Ga, Mg-Se) and the absence of action (Mg-Cis-Pt) may indicate that the sites of anticancer agents and Mg are localized in another part of the cell (Cis-Pt may control the Mg level on N7 of guanine in DNA, for example) if their antitumoral effects are due to competitive actions of Mg.
5.1.2. Magnesium and Anticancer Antibiotic
Mithramycin is an antitumor antibiotic3 that can basically be considered to have powerful properties of the same type as calcitonin. Mithramycin can in fact cause a rapid and severe lowering of the blood magnesium level.
In the human amnion, mithramycin reduces Gt on the maternal side but has no effect on the fetal side. There is no antagonism between Mg and mithramycin. These results limit the role of the membrane in the effects of mithramycin.
5.2. Magnesium and Alcohol
Alcohol may be classified among the carcinogenic or cocarcinogenic factors. Alcohol in vitro, like carcinogenic metals, decreased Gt2. There is a preventive antagonism between Mg and alcohol on the maternal side, and a curative opposing action, independent from Mg concentration, on the fetal side and regardless of Mg concentration on the maternal side81,82.
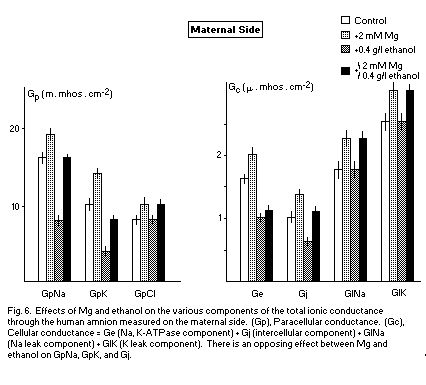
The transamniotic conductance Gt is the sum of various components: paracellular (GpNa, GpK, GpCl) and cellular (Na,K-ATPase component; Ge, intercellular component; Gj, leak component G1Na, G1K).
These various components are reduced by alcohol and increased by Mg. This opposite action may indicate an antagonism between Mg and alcohol on all components of Gt. At a concentration of 2mM (corresponding to a binding state41,42) (Fig. 6) in preventive and opposing action, Mg antagonizes the deleterious effects of alcohol on GpNa, GpK, and Gj on the maternal side. In this case, the membranous effects are important and Mg may be considered as an anticarcinogenic factor.
6. GENERAL CONCLUSIONS
The data described may provide a basis for further developments in the physiological, experimental, and therapeutic fields.
6.1. General Scheme of Relationship Between Magnesium Status and Cancer
Through numerous cellular and systemic mechanisms, the relation between Mg status and cancer appears very complex: both Mg load and Mg deficit sometimes induce anticancer and sometimes carcinogenic effects (Fig. 1-3).
The following general scheme may be retained. Established carcinogenesis induces Mg disturbances which associate tumor Mg load due to Mg mobilization through blood cells with Mg depletion in nonneoplastic tissue. At a premalignant stage and in the case of some hemolymphoreticular malignancies, Mg deficiency seems carcinogenic, but in the case of solid tumors it inhibits tumor development1-3. These various disturbances entail several logical consequences concerning cancer research and treatment.
6.2. Magnesium and Cancer Research
In experimental and clinical cancer and anticancer research, it appears very important to investigate magnesium status and the homeostatic of perturbating factors which regulate or disturb cellular, subcellular, and systemic Mg metabolism. We still have limited knowledge of these processes1-3, but the role of taurine provides a first approach3,37,111-115.
6.3. Magnesium and Cancer Treatment
Indications for cancer therapy of Mg supplementation of of induced Mg deficiency are critical. But it is possible to speculate on the importance of ideal treatment for Mg disturbances in an overall cancer treatment.
6.3.1. Contraindictions and Indications of Mg Therapy
Within the context of our present knowledge, we will consider malignant solid tumors as a relative contraindication of magnesium therapy since their development may be stimulated by Mg. Nevertheless, if Mg depletion induces an important discomfort, one may use palliative Mg therapy to improve the quality of life. This is more permissible as the risk of inducing Mg excess in the sites where it is noxious is prevented--e.g., in the case of efficient cytostatic treatment--by Cis-Pt. Moreover, Mg supply may also decrease both toxicity and anticancer efficiency of the drug1-3,17,116-121, i.e., for cytostatic anthracycline122,123.
Mg supply may be used as an adjuvant treatment in hemolymphoreticular malignancies, particularly when symptomatic Mg deficit appears, aggravated by iatrogenic factors, i.e., use of therapies inducing Mg deficit: cyclosporine for marrow transplantation, aminoglycosides, abdominal irradiation, several cytostatic drugs1-3,17,116-123.
Prophylactic anticancer action requires a balanced, normal Mg metabolism, with sufficient Mg intake in particular1-3.
6.3.2. Induction of Mg Deficit
The induction of severe Mg deficiency through dialysis associated with a diet deficient in Mg, even associated with K deficiency, is the least advisable as it is of dubious efficiency1-3. The use of Mg antagonists that accumulate specifically in tumor tissue would be a more appealing alternative. Gallium would fill this bill but its tolerance and effectiveness are still under study1-3,124,125.
6.3.3. Ideal Treatment
In the future, the ideal treatment for Mg disturbances in cancer should control the systemic and local factors which regulate cellular and subcellular Mg distribution in neoplastic tissues and those which prevent Mg depletion in extramalignant tissues.
ACKNOWLEDGMENT
We thank Dr. Jean-Pierre Mareschi for his valuable help by providing the most recent computerized data for this literature survey.
REFERENCES
1. J. Durlach, M. Bara, A. Guiet-Bara, and P. Collery, Anticancer Res., 6, 1353 (1986).
2. J. Durlach, P. Rinjard, M. Bara, A. Guiet-Bara, and P. Collery, in Magnesium: Physiologische Aspekte für die Praxis (B. Lasserre, ed.), Panscientia Verlag, Hedzingen-Zürich, 1987, p. 26ff.
3. J. Durlach, Magnesium in Clinical Practice, J. Libbey, London, 1988, p. 386.
4. L. J. Anghileri, P. Collery, P. Coudoux, and J. Durlach, Magnesium Bull., 3, 1 (1981).
5. G. Fiskum and R. S. Cockrell, Arch. Blochem. Biophys., 240, 723 (1985).
6. P. Collery, L. J. Anghileri, P. Coudoux, and J. Durlach, Magnesium Bull., 3, 11 (1981).
7. Z. Szmeja and H. Konczewska, H.N.O., 34, 85 (1986).
8. J. Alexandrowicz, J. Dobrowolski, and J. Lisiewicz, Magnesium Res., 2, 83 (1989).
9. M. Abdulla, Z. Gesamte Hyg., 30, 196 (1984).
10. M. J. Laires and J. R. D. Silva, Anticancer Res., 5, 638 (1985).
11. L. Youngman, J. Chen, S. Liu, J. Li, R. Peto, and T. C. Campbell, Fed. Proc., 44, 1341 (1985).
12. A. A. Fomina, Vrach Delo, 0/10, 58 (1986).
13. S. E. Shehin and M. B. Zemel, FASEB J., 2, 3321 (1988).
14. H. L. Bradlow, F. D. Skidmore, M. K. Schwartz, M. Fleisher, and D. Schwartz, in Endocrinology of Cystic Breast Disease (A. Angeli, H. L. Bradlow, and L. Dogliotti, eds.), Raven Press, New York, 1983, P. 197ff.
15. W. Muller and R. Iffland, Acta Neuropathol. (Berl.). 55, 53 (1981).
16. T. G. Frazier, M. E. Mucha, I. H. Rush, E. J. Trull, S. A. Carlson, and J. A. O'Connor, J. Surg. Oncol., 13, 35 (1980).
17. L. Cohen and R. Kitzes, Magnesium, 4, 16 (1985).
18. D. W. Nixon, D. H. Lawson, M. Kutner, J. Ansley, M. Schwarz, S. Heymsfield, R. Chawla, T. H. Cartwright, and D. Rudman, Cancer Res., 41, 2038 (1981).
19. X. Le Loet, C. Ducastelle, J. Hemet, and P. Deshayes, Rev. Rhum. Mal. Osteoartic., 50, 141 (1983).
20. L. F. Belanger, in 1st Int. Symposium on Mg Deficit in Human Pathology; II. Book of Communications and Discussion (J. Durlach, ad.), S.G.E.M.V., Vittel, 1973, P. 425ff.
21. T. Günther, R. Averdunk, K. Wonigeit, and J. Vormann, Magnesium Bull., 10, 22 (1988).
22. S. J. Van Rensburg, J. M. Hall, and D. B. DuBruyn, J. Natl. Cancer Inst., 75, 561 (1985).
23. S. J. Van Rensburg, J. M. Hall, and P. S. Gatherole, Nutr. Cancer, 8, 163 (1986).
24. S. J. Van Rensburg, E. S. Bradshaw, and E. F. Rose, Br. J. Cancer, 51, 399 (1985).
25. S. J. Van Rensburg, S. Afr. Med. J., S21, 9 (1987).
26. G. Macquart-Moulin, E. Riboli, J. Cornee, R. Kaaks, and P. Berthdézène, Int. J. Cancer., 40, 17S (1987).
27. H. Mori, T. Tanaka, and T. Kuniyasu, Magnesium Res., 2, 85 (1989).
28. R. M. Galt, P. A. McCreary, G. H. Laing, and G. M. Hass, in Trace Substances in Environmental Health, Vol. 14 (D. D. Hemphill, ed.), Univ. Missouri-Columbia, 1980, P. 189ff.
29. G. M. Hass, G. H. Laing, R. M. Galt, and P. A. McCreary, Magnesium Bull., 3, 217 (1981).
30. R. M. Galt, G. H. Laing, and G. M. Hass, in Trace Substances in Environmental Health, Vol. 16 (D. D. Hemphill, ed.), Univ. Missouri-Columbia, 1982, p. 205ff.
31. B. J. Mills, R. D. Lindeman, and C. A. Lang, Proc. Soc. Exp. Biol. Med., 181, 326 (1986).
32. R. D. Grubbs, C. A. Wetherill, K. Kutschke, and M. E. Maguire, Am. J. Physiol., 248, C51 (1985).
33. T. Günther, J. Vormann, and R. Averdunk, FEBS Lett., 197, 297 (1986).
34. V. Hoftiezer, P. 0. Berggren, and B. Hellman, Cancer Lett., 27, 7 (1985).
35. T. Günther and J. Vormann, Magnesium Bull., 10, 91 (1988).
36. F. W. Heaton, S. Tongyai, and Y. Rayssiguier, in Magnesium in Health and Disease (I. Itokawa and J. Durlach, eds.), J. Libbey, London, Paris, 1989, P. 27ff.
37. J. Durlach, M. Bara, A. Guiet-Bara, and P. Rinjard, in Magnesium in Cellular Processes and Medicine (B. Altura, J. Durlach, and M. S. Seelig, eds.), Karger, Basel, 1987, p. 219ff.
38. T. Günther, in Magnesium in Health and Disease (Y. Itokawa and J. Durlach, eds.), J. Libbey, London, 1989, p. 3ff.
39. D. L. Guernsey and I. S. Edelman, J. Biol. Chem., 261, 11956 (1986).
40. G. Calviello, D. Bossi, and A. Cittadini, Cell. Biol. Int. Rep., 10, 478 (1986).
41. M. Bara, A. Guiet-Bara, and J. Durlach, Magnesium Res., 1, 23 (1988).
42. M. Bara, A. Guiet-Bara, and J. Durlach, Magnesium Res., 1, 29 (1988)
43. J. E. Trosko and C. C. Chang, J. Am. Coll. Nutr., 7, 428 (1988).
44. B. S. Levine and J. W. Coburn, N. Engl. J. Med., 310, 1253 (1984).
45. M. Bara, A. Guiet-Bara, and J. Durlach, Magnesium Res., 1, 231 (1988).
46. B. L. Vallee, Biofactors, 1, 31 (1988).
47. E. de Vendittis, R. Zahn, and O. Fasano, Eur. J. Biochem., 161, 473 (1986).
48. R. A. Hickie, J. W. Wei, L. M. Blyth, D. Y. Wong, and D. J. Klaasen, Can. J. Biochem. Cell. Biol., 61, 934 (1983).
49. A. Cittadini, F. I. Wolf, G. Calviello, and D. Bossi, Magnesium Res., 2, 126 (1989).
50. S. M. Oredsson, S. Anehus, and O. Heby, Mol. Cell. Biochem., 64, 163 (1984).
51. A. B. Huang, C. M. Lin, and E. Hamel, Biochim. Biophys. Acta, 832, 22 (1985).
52. S. Roychowdhury and F. Gaskin, Biochemistry, 25, 7847 (1986).
53. P. R. Trumbo, J. D. Watkins, and W. G. Martin, Fed. Proc., 41, 362 (1982).
54. J. Widom, J. Mol. Biol., 190, 411 (1986).
55. L. K. Tkeshelashvili, N. V. Sichinava, N. A. Sapozhnikova, and A. N. Rcheulishvili, Biofizika, 28, 582 (1983).
56. S. R. Patierno, M. Sugiyama, J. P. Basilion, and M. Costa, Cancer Res., 45, 5737 (1985).
57. S. R. Patierno and M. Costa, Cancer Biochem. Biophys., 9, 113 (1987).
58. S. R. Patierno, M. Sugiyama, and M. Costa, J. Biochem. Toxicol., 2, 12 (1987).
59. K. Conway, X. W. Wang, L. S. Xu, and M. Costa, Carcinogenesis, 8, 1115 (1987).
60. L. Ungar, Z. Kazy, F. Boldog, J. Menyhart, and P. Siklos, in Actual Standing In EPH Gestosis (C. Goeke, ed.), Elsevier, Amsterdam, 1985, p. 257ff.
61. T. Theophanides and M. Polissiou, Magnesium, 5, 221 (1986).
62. T. Theophanides and M. Polissiou, in Metal-Based Anti-Tumor Drugs (M. Gielen, ed.), Freund, Tel Aviv, 1989.
63. J. E. Morgan, J. W. Blankenship, and H. R. Matthews, Arch. Biochem. Biophys., 246, 225 (1986).
64. S. Devarajan and R. H. Shafer, Nucleic Acids Res., 14, 5099 (1986).
65. M. G. Giles, P. S. Rennie, and N. Bruchovsky, Mol. Cell. Endocrinol., 45, 167 (1986).
66. N. N. Khodarev, I. A. Sokolova, S. S. Aleksandrova, I. I. Votrin, and I. V. Chupyrina, Mol. Gen. Mikrobiol. Virusol. (USSR), 0/9, 24 (1987).
67. J. Nelson, R. Clarke, G. R. Dickson, H. W. Vandenberg, and R. F. Murphy, J. Steroid Biochem., 25, 619 (1966).
68. W. Dietrich, M. Gorlich, and E. Heise, Eur. J. Cancer Clin. Oncol., 22, 181 (1986).
69. I. L. Cameron and N. K. R. Smith, Scan. Electron Microsc., 2, 463 (1980).
70. I. L. Cameron and K. E. Hunter, Cancer Res., 43, 1074 (1983).
71. A. Romeu, L. Arola, and M. Alemany, Cancer Biochem. Biophys., 9, 53 (1986).
72. A. Romeu, L. Arola, and M. Alemany, Oncology, 44, 319 (1987).
73. Z. Chirulescu, C. Chirilolu, A. Suciu, and R. Pirvulescu, Med. Interne (Romania), 25, 257 (1987).
74. Y. Rayssiguier, A. Mazur, P. Cardot, and E. Gueux, in Magnesium in Health and Disease (Y. Itokawa and J. Durlach, eds.), J. Libbey, London, Paris, 1989, p. 27ff.
75. S. Nigam, R. Averdunk, and T. Günther, Prostaglandins Leukotrienes Med., 23, 1 (1986).
76. F. Garaci, R. Paoletti, and M. G. Santoro, Prostaglandins in Cancer Research, Springer Verlag, Berlin, 1987, p. 288.
77. K. Hanada, K. Suzuki, I. Hashimoto, K. Sone, and K. Ishida, Magnesium Res., 2, 19 (1989).
78. R. A. Karmali, T. Wustrow, H. T. Thaler, and R. A. Good, Cancer Res., 44, 467 (1984).
79. E. Bastida, G. Escolar, A. Ordinas, S. L. Giardina, and G. A. Jamieson, J. Lab. Clin. Med., 106, 68 (1985).
80. J. Durlach and Y. Rayssiguier, Revue Alcool. (France), 26, 1 (1980).
81. A. Guiet-Bara, M. Bara, and J. Durlach, Magnesium Bull., 9, 36 (1987).
82. A. Guiet-Bara, M. Bara, J. Durlach, and C. Pechery, Alcohol, 5, 63 (1988).
83. J. Durlach, S. Poenaru, S. Rouhani, M. Bara, and A. Guiet-Bara, in Nutrients and Brain Function (W. B. Essman, ed Karger, Basel, 1987, p. 48ff.
84. K. S. Kasprzak, R. V. Quander, and L. A. Poirier, Carcinogenesis, 6, 1161 (1985).
85. K. S. Kasprzak, J. M. Ward, L. A. Poirier, D. A. Reichardt, A. C. Denn, and C. W. Reynolds, Carcinogenesis, 8, 1005 (1987).
86. A. S. Prasad, in Zinc Metabolism (A. S. Prasad, ed.), C. C. Thomas, Springfield, IL, 1966, p. 288ff.
87. M. Bara, A. Guiet-Bara, and J. Durlach, J. Trace Elem. Electrolytes Health Dis., 2, 111 (1986).
88. J. Durlach, Rev. Franc. Allergol., 15, 3 (1975).
89. F. Gaudin-Harding, Magnesium Bull., 3, 229 (1981).
90. J. H. McCoy and M. A. Kenney, in Magnesium in Cellular Processes and Medicine (B. M. Altura, J. Durlach, and M. S. Seelig, eds.), Karger, Basel, 1987, p. 196ff.
91. R. A. Rudders and J. Andersen, Proc. Soc. Exp. Biol. Med., 174, 33 (1983).
92. K. Nakanishi, B. Zbar, T. Borsos, and G. Glenn, Cancer Res., 46, 3886 (1986).
93. E. L. Pryzdial and D. E. Isenman, Mol. Immunol., 23, 87 (1986).
94. K. E. Muse and H. S. Koren, J. Reticuloendothel. Soc., 32, 323 (1982).
95. A. Lichtenstein, Blood, 67, 657 (1986).
96. E. Maly, Med. Hypotheses, 11, 177 (1983).
97. M. K. Horwitt, J. Nutr., 116, 1371 (1986).
98. A. Nishio, S. Matsumoto, S. Ishiguro, and M. Noburu, Gakujutsu Hokoku-Kagoshima Daigaku Nogakubu, 35, 189 (1965).
99. C. Burtin, C. Noirot, P. Scheinmann, L. Galoppin, D. Sabolovic, and P. Bernard, Eur. J. Cancer Clin. Oncol., 22, 161 (1988).
100. C. Burtin, Le Concours Médical, 111, 2 (1989).
101. B. Beutler and A. Cerami, N. Engl. J. Med., 316, 379 (1987).
102. P. Maurois, Y. Rayssiguier, E. Gueux, and E. Deicase, Magnesium Res., 1, 118 (1988).
103. S. M. Ribeiro and H. A. Armelin, Mol. Cell. Biochem., 59, 173 (1984).
104. T. Günther, J. Vormann, and E. Liss, Magnesium Bull., 7, 1 (1985).
105. M. Terasaki and H. Rubin, Proc. Natl. Acad. Sci. USA, 82, 7324 (1985).
106. W. L. McKeehan, Fed. Proc., 43, 113 (1984).
107. R. Cariou and G. Tobelem, Presse Médicale, 17, 2219 (1988).
108. D. R. Buck and J. Bales, J. Nutr., 113, 2421 (1983).
109. S. Cohen, Cancer, 51, 1787 (1983).
110. T. Koike, Brain Res., 299, 293 (1983).
111. M. Hashimoto, E. Nishida, and H. Sakai, Zool. Sci., 1, 195 (1974).
112. F. Franconi, F. Martini, I. Stendardi, R. Matucci, L. Ziletti, and A. Giotti, Biochem. Pharmacol., 31, 3181 (1982).
113. M. Masuda, K. Horizaka, and T. Koeda, Nippon Yakurigaku Zasshi, 84, 283 (1984).
114. C. E. Wright, T. T. Lin, Y. Y. Lin, J. A. Sturman, and G. E. Gaull in Taurine: Biological Actions and Clinical Perspectives (S. S. Oja, L. Ahtee, P. Kontro, and M. K. Paasonen, eds.), Alan R. Liss, New York, 1985, p. 137ff.
115. H. F. Pierson, J. M. Fisher, and M. Rabinovitz, J. Natl. Cancer Inst., 75, 905 (1985).
116. N. J. Vogelzang, J. L. Torkelson, and B. J. Kennedy, Cancer, 56, 2765 (1985).
117. D. L. Trump and L. Horvet, Cancer Treatment Rep., 69, 259 (1985).
118. A. F. Stewart, T. Keating, and P. E. Schwartz, Am. J. Obst. Gynecol., 153, 660 (1985).
119. J. H. M. Blom, K. H. Kurth, and T. A. W. Splinter, Int. Urol. Nephrol., 17, 331 (1985).
120. J. C. Willox, E. J. McAllister, G. Sangster, and S. B. Kaye, Br. J. Cancer, 54, 19 (1986).
121. D. Daugaar, S. Strandgaard, N. H. Holstein-Rathlou, P. L. Frederiksen, U. G. Svendsen, O. Munck, and F. P. Leyssac, Scand. J. Clin. Lab. Invest., 47, 455 (1987).
122. Z. H. Lin, S. G. Li, J. J. Ye, and Y. Zhang, in Magnesium in Health and Disease (Y. Itokawa and J. Durlach, eds.), J. Libbey, London, Paris, 1989, p. 53ff.
123. T. Okazaki, T. Mochizuki, M. Tashima, H. Sawada, and H. Uchino, J. Cell. Physiol., 131, 50 (1987).
124. P. Collery, H. Millart, M. Pluot, and L. J. Anghileri, Anticancer Res., 6, 1085 (1986).
125. R. P. Warrel, Jr., M. Issacs, N. W. Alcock, and R. S. Bockman, Ann. Intern. Med., 107, 683 (1987).


No comments:
Post a Comment