In Adjuvant Nutrition in Cancer Treatment, Eds. P. Quillan and R. M. Williams. Publ Cancer Treatment Research Foundation, 1993. Chapt. 15:238-318.
MAGNESIUM IN ONCOGENESIS AND IN ANTI-CANCER TREATMENT: INTERACTION WITH MINERALS AND VITAMINS
Mildred S. Seelig, M.D., M.P.H.
Adjunct Professor, Department of Nutrition, School of Public Health, North Carolina University Medical Center, Chapel Hill, N.C.
The section headers of this paper are as follows:
- I. INTRODUCTION
- II. EPIDEMIOLOGIC CORRELATION OF MAGNESIUM WITH CANCER
- III. MAGNESIUM IN EXPERIMENTAL ONCOGENESIS
- IV. POSSIBLE MECHANISMS INVOLVED IN MAGNESIUM EFFECTS ON CANCER
- V. MAGNESIUM LOSS-ASSOCIATED TOXICITY OF ANTINEOPLASTIC AGENTS
- VI. CONCLUDING COMMENTS
- REFERENCES
I. INTRODUCTION
Magnesium (Mg) deficiency can paradoxically increase the risk of, or protect against oncogenesis.(1) Over 300 enzymes that influence the metabolism of carbohydrate, amino acids, nucleic acids and protein, and ion transport, require Mg.(2,3) Its roles in fatty acid and phospholipid acid metabolism, that affect permeability and stability of membranes, are being elucidated.(4-6) It has been proposed that Mg is central in the cell cycle, and that its deficiency is an important conditioner in precancerous cell transformation.(7-9) In addition, immunocompetence (that eliminates transformed cells) is Mg-dependent.(1,10-12) Mg supplementation of those who are Mg deficient, like chronic alcoholics, might decrease emergence of some malignancies.(13) Epidemiologic studies suggest that low water and soil Mg may predispose to some cancers, but not to others.(14-18) Hard water is directly related to longevity, but the age-associated cell mutation and diminished capacity for immunosurveillance can obscure geochemical effects.(19,20) Lympholeukemia(11-13,21-29) and bone tumors,(30-33) resembling those seen in children, have developed in some normally resistant rat strains when Mg deficiency was begun at weaning, but not in others. The effect of Mg on cancer produced by tumor transplants, or by chemicals, has depended on the time Mg supplementation or deficiency was induced, relative to exposure to oncogens.(12-13) Optimal Mg intake may be prophylactic against initiation of some neoplasms. Since cancer cells have high metabolic requirements, it is not indicated (alone) in the treatment of cancer.(13)
II. EPIDEMIOLOGIC CORRELATION OF MAGNESIUM WITH CANCER
A. INCREASED LONGEVITY IN HARD WATER AREAS
Since environmental factors have been judged likely to contribute to most human cancers,(34) it is worth effort to ascertain if there are protective geochemical agents. Determining what it is in different geographic regions, that affects life expectancy, provides one approach. The largest area in the United States of America (USA) with increased longevity is in the north and central plains; the largest area with decreased longevity is in the south-eastern coastal area.(35) These are hard and soft water regions, respectively.
B. PREMATURE CARDIOVASCULAR DEATHS AND WATER HARDNESS
Greater morbidity and mortality from cardiovascular disease is directly correlated with water softness and diet.(36-40) Metabolic balance studies, with normal young adults on their usual diets, show that the lesser American Mg intake by adults, causing negative Mg balance, than in the Orient,(39) correlates with the much higher death rate from ischemic heart disease (IHD) in the USA.(38,40) Most American diets provide less than 70% of the 1980 recommended dietary allowance (RDA) of Mg.(41-45) Experimental and clinical studies, and epidemiologic findings indicate that it is Mg, rather than Ca, that protects against IHD, myocardial infarcts and sudden unexpected cardiac death caused by arrhythmias.(40,45-54)
C. INCREASED RISK OF CANCER WITH AGING
Cancer is second to heart disease as a cause of death in the aged, and thus is more common in regions where more people reach old age. Depressed B-cell and T-cell immunologic function, occur with aging.(55-57) Also, the longer the exposure to environmental agents with oncogenic potential, the greater the risk of developing cancer. Thus, the increased longevity of those living in hard water areas might obscure protection by geochemical factors against cell transformation. Neoplasms of children, that end life early, can contribute to decreased longevity (i.e. in soft water regions). The effect of geochemical factors is difficult to interpret, since such oncogenic trace metals as cadmium (Cd), lead (Pb), and nickel (Ni) are found more in soft than in hard water.(19,34)
D. CANCER RATES IN LOW MAGNESIUM REGIONS
An inverse relation between cancer prevalence and the Mg content of water and of soil and cancer was reported from worldwide early studies, starting more than 50 years ago (Table 1(14,59-63)).
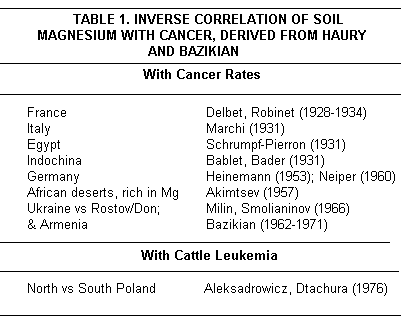
Bazikian(14) reviewed more recent studies (Table I(64-68)). A Russian report showed that stomach cancer is four times more common (40/100,000) in the Ukraine where the Mg content of soil and drinking water is low, than it is in Armenia (10/100,000) where the Mg content is more than twice as high.(14,66-68) A more recent morphologic and statistical analysis of neoplastic deaths in two Polish communities(69) disclosed a nearly three-fold higher death rate in the one in a low soil Mg area (27%) than in the one with high soil Mg (10%). The malignancies accounting for the differences were mainly adeno- and squamous cell carcinomas in the gastrointestinal tract (61.3%) and respiratory system (22.3%). Armstrong70 criticized such correlations, because other environmental factors are usually ignored. Aleksandrowicz et al,(15-18) in Poland, also considered aflatoxins from environmental fungi, radiation, use of pesticides, and application to the soil of fertilizers that are rich in phosphates, potassium (K) and nitrogen (that lower Mg and selenium [Se] in the earth), in evaluating regional differences in leukemia. They conclude that inadequacy of Mg and antioxidants are important risk factors in predisposing to leukemias.
Correlation of high rates of leukemia with low levels of Mg in soil and water is concordant with experiments showing that chronic Mg deficiency can cause lymphosarcomas and leukemia in rats.(10,13,21-29) Inhabitants of regions with high soil Mg content in Poland, have much lower leukemia rates than do residents of regions where the amount of available Mg is low.(18) The incidence of leukemia is lower in the Orient than in several European countries: Scandinavia, Scotland, England, Wales, Belgium, Holland, Switzerland, and in the USA (71). As with the human disease, cattle leukemia is rare in the Orient and more frequent in Denmark, Sweden and the Baltic.(18) In contrast to these findings is the lesser frequency of leukemia in southeastern (soft water) USA, than in the northwest (also a soft water region), and higher in the north midwest, where the water is hard.(72) Water can contribute 0.4 to 300 mg/d of Mg: i.e. soft waters in southeast states provided only 0.35 to 0.84 mg/d, whereas the Mg RDA is almost met by the hardest waters.(73) The disparity between American and European findings warrants study.
III. MAGNESIUM IN EXPERIMENTAL ONCOGENESIS
A. SPONTANEOUS NEOPLASMS IN MAGNESIUM DEFICIENT RATS
1. Lymphomas and Leukemias
Subacute Mg deficiency has caused lymphopoietic neoplasms in young rats. A study of rats surviving Mg deficiency sufficient to cause death in convulsions during early infancy in some, and cardiorenal lesions weeks later in others, disclosed that some of survivors had thymic nodules or lymphosarcoma.(22)Extension of the studies showed that 20% of 92 rats of two strains, which rarely have spontaneous lympholeukemia, had thymic lymphosarcoma after 65 days of Mg deficiency.(23,24) None on the same diet with Mg added developed the tumor. The vulnerability of Wistar and Sprague-Dawley rats to Mg deficiency induced lymphosarcoma and terminal lymphatic leukemia, was confirmed by others.(25-28) Susceptibility of young rats to transmission by viable lymphoma cells was intensified by Mg deficiency.(29) Rat strain variability in vulnerability was then found: 25-50% of Holzmann rats developed thymic tumors; Fischer and Osborne-Mendel rats were resistant. Chronic myeloid leukemia, following persistent neutrophilic leukocytosis, such as appears in acutely Mg deficient rats,(74,75) developed in 10% of susceptible rats with chronic Mg deficiency. Normal Mg intakes reversed the leukocytosis, except for those progressing to irreversible myeloid leukemia (Table 2).(25-28)
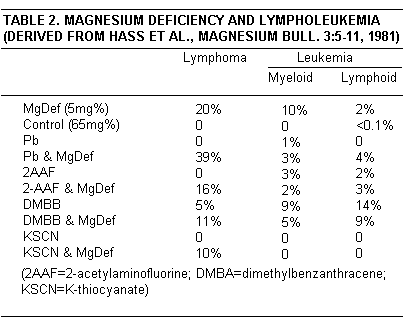
The age at which Mg deficiency is induced, its degree, and duration determine the percentage incidence and the type of neoplasm.(11-13) Thymic lymphosarcoma develops in susceptible strains of rats provided diets with 5 mg% Mg at or soon after weaning and lowered to 3 mg% at the 6th-24th week of life: 20% when Mg the inadequate diet was started at 25-35 days of age, 10%. when started at 35-60 days, 0 when started at 120 days and older. Thymic atrophy was seen in young and older rats that did not develop the neoplasm during the subacute phase. During Mg deficiency for 24 weeks, chronic myeloid leukemia, which is never seen in Mg-adequate vulnerable strains without use of oncogenic agents, developed in 10%.
The influence of high Ca/Mg ratios (that are common in stock rat diets), on lymphoid proliferation, is considered below. Casting doubt on the speculated role of inadequate antioxidants as a factor in the high rates of lympho-leukemias in low Mg regions,(18) are the recently reported findings by Guenther et al(76) that Mg deficient rats that were vitamin E supplemented developed more thymic lymphomas than did those on diets that were also low in vitamin E.
2. Bone Tumors
Osteomyelosclerosis (comparable to that seen in some leukemia patients) and sub-periosteal desmoid tumors have developed in intact and parathyroidectomized Mg deficient rats.(30-33) The characteristics of the bone overgrowth were midway between hyperplasia of connective tissue and a true tumor.(77-79) The tumor had many mitotic figures in fibroblasts, suggesting accumulation of cells not able to differentiate normally. Mg repletion produced rapid disappearance of the periosteal tumor)s.(79) Osteogenic precursor cells failed to differentiate fully when bone matrix was implanted intramuscularly in Mg deficient rats.(80) Decreased phosphatases, matrix, collagen, and bone Mg/Ca ratio was found in tumor-like femoral exostoses in 10 of 11 Mg deficient rats in another study.(81) Mg was needed for cartilage cell maturation.
3. Intestinal Tumor-Like Overgrowth
Connective tissue, made up of fibroblastic cells that produced collagen type III, proliferated in the intestines of rats maintained on severely Mg deficient diets for at least 8 weeks.(82) A less Mg-restricted diet did not evoke such tumors.(76)
B. MAGNESIUM EFFECT ON CHEMICAL INDUCTION OF OR ESTABLISHED NEOPLASMS
1. Chemical Carcinogens
The effect of Mg deficiency or administration on induction of tumors by chemical agents, or on growing neoplasms, is not uniform. Both inhibition and enhancement of skin cancer caused by application of tar to skin of rodents has been caused by Mg.(59,83) Mg deficiency increased survival of rats given Flexner-Jobling carcinoma transplants; adequacy of dietary Mg had no effect on tumor growth, but large Mg supplements accelerated growth of the established carcinoma.(84) Murine sarcoma-growth was increased by high dosage Mg; melanomas were not affected. Mg deficiency, induced in rats with palpable mammary adeno-carcinomas, decreased tumor growth.(85) This effect has recently been confirmed in athymic nude mice, made Mg deficient before and after inoculation with a human mammary cancer cell line.(86) In contrast, rat sarcoma and rabbit Brown-Pearce carcinoma developed more slowly in animals fed Mg salts than in those that were Mg deficient, as did Ehrlich ascites tumor.(87) Injection of Mg to mice, before thiophosphamide, reduced its oncogenic effects, and lengthened survival time.(88) In the extensive studies by Hass et al(11-13), Mg deficiency intensified rat leukemia induced by weak agents; its effect on induction of solid tumors by carcinogens was not uniform (Table 3).
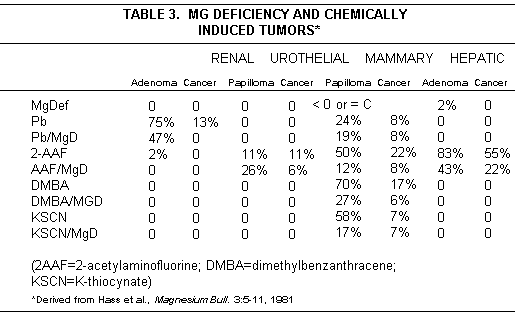
It has recently been shown that Mg supplementation inhibited the increased DNA synthesis of the colon epithelium, excess proliferation of which had been chemically induced in rats; it was suggested that this effect might be related to Mg suppression of oncogen-induced large bowel carcinogenesis.(89)
2. Magnesium and the Oncogenicity of Lead, Nickel and Cadmium
Lead (Pb) salts, are more leukemogenic when given to Mg deficient rats, than when they are given to Mg-adequate rats,(12) suggesting that Mg is protective. Pb-induced pulmonary adenomas in mice, were prevented by Ca or Mg salts given simultaneously.(90) Induction of pulmonary adenomas by Pb and nickel (Ni) salts in mice was also counteracted by Ca or Mg salts.90 Subcutaneously injecting or feeding Mg and/or Ca salts to rats given a Ni salt intramuscularly did not alter emergence of sarcomas; admixture of Mg (but not Ca) salt with the Ni salt reduced the number of rats with tumor formation: from 85% to 25%.(91) It was suggested that Mg inhibits Ni carcinogenesis, probably in part from Mg-antagonism of suppression by Ni of the T cell killer activity.(92-94) Mg may also protect against Ni oncogenicity by inhibiting Ni-induced breaks in deoxyribo-nucleic acid (DNA) strands, that gives rise to abnormal chromosomes and cell transformation.(95) Feeding Mg salts to rats did not modify the carcinogenicity of Cd, but admixture of Mg with Cd prevented sarcoma formation at injection sites.(96) Studies of the interrelations of Mg with carcinogenic metals, using the amniotic membrane,(97) has indicated that its protective effect against the weak oncogens Pb and Cd, is as a competitive antagonist at the membrane level. Mg is a non-competitive antagonist of Ni, exerting its effect in the nucleus.(97)
IV. POSSIBLE MECHANISMS INVOLVED IN MAGNESIUM EFFECTS ON CANCER
A. INTERRELATIONS OF MAGNESIUM AND CALCIUM IN ABNORMAL CELL METABOLISM
1. Ca/Mg Ratio Effects on Thymic Changes
The diets used in studies of Mg deficiency-induced lympholeukemia in rats had an extremely high Ca/Mg ratio: 140/1, causing both depletion of Mg and hypercalcemia.(92) Excess Ca stimulates mitosis of thymic and bone marrow cells strongly.(99) A tenfold lesser dietary Ca/Mg ratio of 14/1 caused thymic hypertrophy, but not thymic malignancy, six or more weeks after Mg deficiency had been started in a third of young rats, and in 16% of older rats; it was preventable by Mg.(92) Thymuses that were not hypertrophic were atrophic. That Mg depletion, rather than Ca excess, causes the neoplastic change is suggested by the lymphosarcoma seen in 1 of 4 Mg deficient parathyroidectomized rats, that were hypocalcemic.(22) Cultured lymphocytes exposed to different Ca++ concentrations exhibit increased DNA synthesis on exposure to excessive Ca++, and profoundly suppressed DNA synthesis in low Ca++ concentrations.(100) Low Mg++ in the media were less inhibitory of DNA synthesis.(101) Culture of lymphocytes from humans in Mg-deficient media, resulted in morphologically and functionally abnormal cells;(102) cells with increased oncogenicity emerged when mouse lymphoma cells were cultured in media low in both Mg and Ca.(103) Malignant T-cells derived from thymic lymphomas induced by 14 weeks of Mg deficiency (in both Lewis and Wistar rats) have 3-fold increased Ca++.(16,104) The murine lymphoma cell clone is a good model for Mg deficiency; T-lymphocytes isolated from rats made Mg deficient for 8 weeks had characteristics similar to those of the mouse lymphoma cells; both had growth abnormalities, depressed hormonal responses, and marked alteration in receptor-G-protein coupling.(105)
2. Mg/Ca Interrelations in Cell Cycle
Mg, which has a central regulatory role in the cell cycle, that affects transphorylation and DNA synthesis, has been proposed as the controller of cell growth, rather than Ca.(106-108) This has been related to mitosis and division.(7-9) It is postulated that Mg++ controls the timing of spindle and chromosome cycles by changes in intracellular (i.c.) concentration during the cell cycle: i.c. Mg falls as cells enlarge, until it reaches a level that allows for spindle formation. Mg influx then causes spindle breakdown and cell division. Thus: 1. Mg, not Ca, controls key rate-limiting steps in the cell cycle at the onset of DNA synthesis and mitosis, a function that may be lost in transformed cells, and 2. processes thought to be regulated by Ca/calmodulin are Mg-dependent, since low i.c. Ca levels can be regulators only when there is adequate free Mg. The metabolic effects of Ca are produced indirectly through its competition with Mg for membrane sites.(107)
Activators of lymphoblastic mitosis, mediated by stimulation of Mg-dependent adenylate cyclase, increase cyclic adenosine monophosphate (cAMP) levels - causing Mg influx which initiates mitosis.(109) Changes in i.c. Mg-binding is associated with different degrees of transformation. If membrane receptors that control Mg are altered by transformation, or if there is continuous exposure to oncogens, which stimulate cell division, cells may revert to a primitive state, and tumors may result when growth and division is limited only by available nutrients.(8) Membrane inositol lipids, influenced by Mg at several steps, have also have been implicated in the action of oncogenes.(9) Differentiation of promyelocyte leukemia HL-60 cells (induced by calcitriol, trans-retinoic acid, or other agents) has been shown to be inhibited by exclusion of Mg from the suspension medium.(110) Incubation of the cells in Mg-adequate medium Mg induced differentiation.
Evaluation of the data on the role of Mg in cell proliferation indicates absolute requirements in crucial steps of cell activation that can trigger normal and neoplastic cell division, but the precise phase(s) of cell cycle where Mg2+ exerts its regulatory effects is in need of further study.(111,112) The i.c. Mg, which is present in a small amount as the free cation, or bound to ligands (i.e. the many enzymes it activates), is compartmented to a different degree in different types of cells; it is not influenced by extracellular (e.c.) Mg concentration.(112) In some cell types rapidly and slowly or non-exchangeable compartments are detectable. In gradually Mg depleted cells, Mg2+-dependent metabolic functions are inhibited in the following order: glycolysis < RNA and DNA synthesis < respiration < protein synthesis protein synthesis appears to be the most sensitive function affected.(113)
B. MEMBRANE/ENZYME STUDIES OF MAGNESIUM DEFICIENCY
Altered membrane phospholipids influence the viscosity of membranes, which is decreased both in Mg deficient and in cancer cells. Membrane abnormalities of erythrocytes of Mg deficient rodents were shown to be responsible for their reduced survival time.(114) Mg low cell membranes have recently been shown to be characterized by increased fluidity and permeability.(4,5) Changed lipid membrane components can reflect changes in lipid metabolism caused by Mg deficiency.(115) Lowered cholesterol and sphingomyelin, and decreased ratios of sphingomyelin/ phosphatidyl-choline and cholesterol/phospholipid result from Mg deficency.(4) Heaton and Rayssiguier propose that membrane changes of Mg deficiency are caused by altered binding to negatively charged phosphate groups of phospholipids of the membranes.(4) Guenther et al(6,116,117) have shown that Mg deficiency induced lymphoma cells also have such membrane lipid changes: higher phosphatidyl-inositol and phosphatidyl-choline and decreased cholesterol, which cause decreased membrane viscosity. There was also abnormal enzyme activity affecting phospholipids.(118)
Anghileri et al(119-121) propose that modifications of cell membranes are principal triggering factors in cell transformation leading to cancer. Using cells from induced cancers, they found that there is much less Mg++ binding to membrane phospholipids of cancer cells, than to normal cell membranes. This might be involved in precancerous changes; in the preneoplastic phase, binding of Mg to i.c. membranes is decreased at the same time that cytosolic Mg increases. There is drastic change in ionic flux from the outer and inner cell membranes (higher Ca and Na; lower Mg and K levels), both in the impaired membranes of cancer, and of Mg deficiency. It has been suggested that Mg deficiency may trigger carcinogenesis by altering fidelity of DNA replication, and increasing membrane permeability.(122) A mechanism proposed is competition of Mg++ with oncogens for DNA binding sites, and its prevention of incorporation of incorrect nucleotides during DNA synthesis. Abnormal membrane properties in Mg deficient lymphocytes may give rise to defective differentiation that can lead to lymphomata.(6,103,104) The membranes have a smoother surface than normal, and decreased membrane viscosity, analogous to changes in human leukemia cells.(100,123)
On the other hand, malignant tumors have higher Mg levels than do normal tissues,(97,124-129) possibly caused by the "capture" of Mg by the tumor,(13) as a result of the high Mg requirement of growing cells. There is resultant lowering of Mg levels in healthy tissues.(13,97)
C. MAGNESIUM AND IMMUNOSURVEILLANCE
Undernutrition causes both humoral and cell mediated immuno-incompetence,(130) as does aging.(55-57) In view of the lymphoid changes caused by Mg deficiency, not surprisingly, it depresses cell-mediated immunity. It impairs phagocytic activity, as well as lymphocytic function.(131) Loss of cell-mediated immunocompetence of Mg deficient rats is considered responsible for the increased "takes" of transplanted lymphoma cells and loss of capacity to be immunized against transplanted lymphoma cells.(28,29) This group suggests that lympholeukemia of Mg-low rats, which resembles the disease of children, may be a consequence of Mg-induced failure of hepatic synthesis and storage of maturation factors; Mg-deficiency lymphoma has been prevented by a substance in a liver powder from Mg adequate rats, that was missing from livers of Mg deficient rats.(10,11)
Immunologic defects of lymphoma cells include reduction of concavalin A stimulation of phospholipid metabolism, and of thymidine uptake and IgG synthesis,(117) and failure to synthesize interleukins, which are related to proliferation and function of lymphocytes.(6,132,133) In addition, cytoxic T-cells, which lyse aberrant cells have defective response to antigens in Mg-deficient media;(132,133) this is consistent with development of lympho-leukemias in Mg deficient rats, and with their failure to reject transplanted lymphoma cells.(28,29) Mg deficiency-induced lymphoma cells also lack a physiologic growth factor and produce an abnormal one, that may participate in the malignant process.(104)
1. Interactions of Magnesium, Zinc and Pyridoxine
The metabolism of Mg is interlinked with that of pyridoxine,(134-137) (Figure 1) and with that of Zn - which is also interlinked with vitamins B6 and E. Deficiencies of each can contribute to cancer. (Figure 2)(1,135)
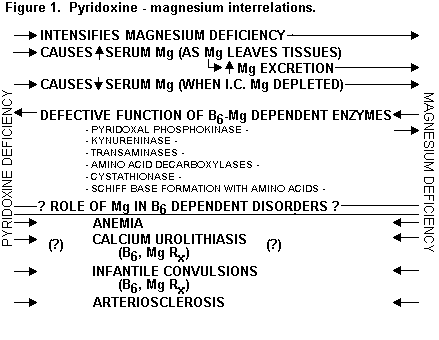
Figure 2: INTERRELATIONS AMONG MAGNESIUM, ZINC, VITAMINS B6 AND E
Mg And Zn -- Each Needed for many enzyme systems; both for some systems
Each needed for nucleic acid and protein synthesis
B6 Deficiency-- Signs similiar to those of Mg deficiency; response to Mg
Decreased cellular Mg And Zn levels
Decreased oxidative phosphorylation (Mg dependent)
Abnormal tryptophan metabolism (Mg dependent enzymes)
Mg and vitamin E
Vitamin E -- anti-oxidant; free-radical scavenger
With Mg, stabilizes cell membranes
Deficiency of E lowers tissue Mg levels
Mg And Zn -- Each Needed for many enzyme systems; both for some systems
Each needed for nucleic acid and protein synthesis
B6 Deficiency-- Signs similiar to those of Mg deficiency; response to Mg
Decreased cellular Mg And Zn levels
Decreased oxidative phosphorylation (Mg dependent)
Abnormal tryptophan metabolism (Mg dependent enzymes)
Mg and vitamin E
Vitamin E -- anti-oxidant; free-radical scavenger
With Mg, stabilizes cell membranes
Deficiency of E lowers tissue Mg levels
Pyridoxine deficiency causes loss of tissue Mg, and its supplementation increases Mg tissue uptake.(3,134-137) Pyridoxine deficiency also adversely affects Zn metabolism by decreasing its absorption,(138) and lowering its tissue levels.(139) All participate in cell-mediated immunity.(1,130-133,135,136,153-158)
2. Vitamin B6, Zinc, Immunity and Cancer
Pyridoxine deficiency, like that of Mg, causes thymic depletion, as well as decreased cell-mediated and humoral immune response.(141,142) T- and B-cells are more affected by pyridoxine deficiency than are killer cell and macrophage function. Low plasma pyridoxal phosphate levels and abnormal tryptophan metabolites: 3-OH-anthranilic acid (OHA) and 3-OH-kynurenine (indicative of disturbed B6 metabolism,(143,144) that have been detected in patients with cancers of breast and bladder,(145-151) and of intestines and lungs,(144) are evidence of vitamin B6 deficiency in patients with malignancies. Bladder tumors recurred in patients with abnormal tryptophan metabolism.(146) Abnormal tryptophan metabolites, as are produced by pyridoxine deficiency, have induced bladder cancer in animals.(152) In view of the interrelations of Zn and/or Mg in pyridoxine metabolism, might deficiency of one or both be contributory? A family prone to bladder cancer was found to have high levels of an oncogenic tryptophan metabolite, that was associated with abnormal kynureninase (a B6-dependent enzyme), although pyridoxine deficiency was not manifest.(150)Since that enzyme is also Mg-dependent, a genetic Mg abnormality might be a factor.
Clinical and animal Zn deficiency impairs T-cell mediated immunity, predominantly, although B-cells are also affected (Figure 3).(153-158
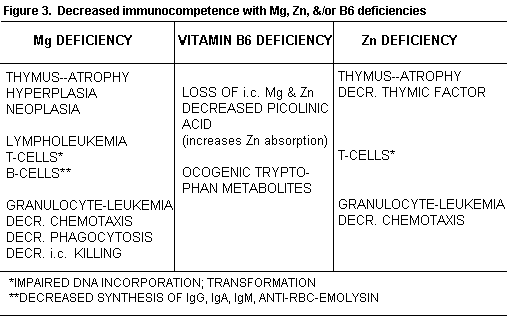
Patients with Zn loss caused by alcoholism, intestinal inflammatory disease or other causes of malabsorption, also are deficient in Mg, and are prone to development of cancer.(1) Both cations are required for synthesis of nucleic acids and enzymatic activity, and their deficiency causes impaired immunosurveillance. Zn deficiency has increased oncogenicity of some chemical agents and decreased that of others.(159,160)
3. Vitamin E, Magnesium, Immunity and Cancer
Membrane damage caused by agents that increase free radicals and peroxidize lipids has been implicated in the decreased immunocompetence of the aged.(161,162) Low serum levels of vitamin E, an anti-oxidant, have been associated with high incidence of lung cancer.(163) Membrane lipid abnormalities in Mg deficient and in neoplastic cells(9,115-119) involve peroxidation of unsaturated fatty acids. Protection of membranes by antioxidants, such as vitamin E, deficiency of which has intensified Mg deficiency in rats,(164) and lowered tissue Mg levels(165-167) may be a means by which antioxidants may protect against cancer.
V. MAGNESIUM LOSS-ASSOCIATED TOXICITY OF ANTINEOPLASTIC AGENTS
A. IRRADIATION
Mg depletion from intestinal Mg loss and malabsorption has contributed to death caused by total body irradiation.(162) Increased Mg intake has protected rodents from whole body irradiation.(162,129) Irradiation of the intestines, while treating cancer of abdominal or pelvic neoplasms, causes hypomagnesemia.(162,170-172) Correction of radiation-induced proctosigmoiditis by MgSO4 infusions in ten patients, in contrast to its persistence in another comparable group of ten patients not so treated suggests the utility of this approach.(173)
B. CHEMOTHERAPEUTIC DRUGS
1. Cyclophosphamide; Dimethylbenzathracene (DMBA)
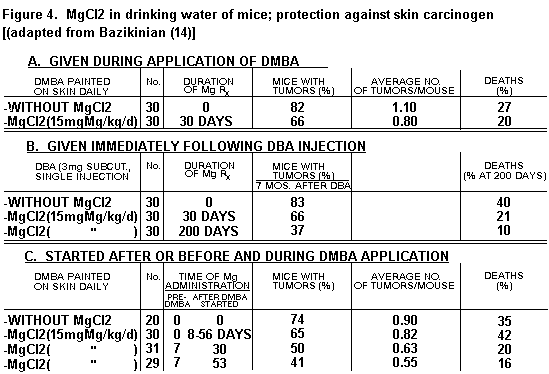
Toxicity of cyclophosphamid in mice was significantly decreased by Mg, with or without Ehrlich's ascites tumor cells.(1,14) Administration of Mg salts daily had some protective effect against tumors produced by application to the skin or injection of DMBA when started the day the oncogen was started, but not when started later (Figure 4).
2. Cisplatin and Other Antineoplastic Drugs
Hypomagnesemia, caused by cisplatin-induced renal tubular defect in Mg reabsorption, that persists long after the drug treatment has been stopped, has been identified in cancer patients.(174-179) The renal tubular lesion seems to be of the distal convoluted segment, like that of Bartter's syndrome.(176) Comparable renal tubular wasting occurs with use of vinblastine and bleomycin(175) and with cyclosporin.(178) The observation of progressive lowering of blood Mg levels, despite gradual correction of urinary Mg loss over the week after a single dose (given to patients with lung cancer), suggested that besides damage to tubular function, cisplatin may also interfere with Mg cellular metabolism.(180) That the drug-induced Mg loss can cause arrhythmias in cancer patients, even during the first cycle of treatment (with cisplatin and fluoro-uracil) was demonstrated in 32 Holter monitored patients.(181) Preventing the lowering of Mg levels by intravenous (i.v.) Mg infusions has prevented adverse effects of hypomagnesemia.(178,182,183) The anticancer activity of cisplatin has been linked to its effects on mitochondrial Mg(184), and to nucleic acid Mg.(185) Since tumor growth has been enhanced supplementation with Mg (supra vide), preventing or correcting the induced Mg depletion, it was anticipated that Mg administration to cancer patients under treatment might diminish the efficacy of the antineoplastic regimen.1 However, clinical experience has obviated that apprehension: Mg supplementation, accompanying cisplatin treatment, has not affected tumor growth rates in human patients(178,182,183), an effect that was confirmed in mice with induced fibrosarcoma or leukemia, treated with cisplatin with and without Mg protection against nephrotoxicity.(186) The results of a 14 month randomized prospective trial indicates that i.v.Mg supplementation should be given during the antineoplastic courses, with oral Mg supplementation provided between courses.(182)
3. Other Magnesium-Wasting Drugs in Cancer Treatment
Renal Mg wasting caused by gentamicin has intensified that caused by anti-neoplastic drugs.(187) Amphotericin B binds Mg to cell membranes, inactivating it,(188) thereby intensifying Mg deficiency.
C. INDUCED MAGNESIUM DEFICIENCY TO TREAT CANCER
Since antineoplastic regimens employ Mg-wasting agents, and rapidly metabolizing cancer cells have high Mg requirements, use of iatrogenic Mg depletion to treat inoperable cancers has been tried, and found effective,(189,190) but associated with serious consequences: acute tumor necrosis resulting in hemorrhage, arrhythmias and stroke. Recurrence of tumor with Mg repletion necessitates surgical removal of remaining tumor and antineoplastic chemotherapy or radiation.
Diminishing Mg in cancer tissues without causing Mg depletion, i.e. by competitive inhibition of Mg within the cancer is preferable, if feasible.(137,191-193) Gallium's antineoplastic activity has been attributed to its replacement of Mg in tumors.(191-195) Search for compounds, that preferentially deplete cancer cell Mg might be fruitful.
VI. CONCLUDING COMMENTS
Epidemiologic and experimental data suggest that adequate Mg might protect against initiation of precancerous cellular changes. However, although increased longevity in Mg-rich geographic areas is clearly correlated with lower heart disease mortality,(36-38,40,46-54) its correlation with cancer prevalence is inconsistent.(1,13-18,58-72) The risk of most cancers increases with aging, and with length of exposure to agents with oncogenic potential. This confounds interpretation of geochemical data. For example, prevalence of bovine and human lympholeukemias in areas of low soil Mg in Poland,(15-18) is in accord with the rat studies implicating low Mg in these malignancies, but this is not true in the USA.(72) What are the oncogenic factors in the hard water (high Mg) American midwest that might predispose to leukemia so as to negate protective effects of Mg? The lower incidence of gastric cancers in high Mg than in low Mg areas might also reflect a protective effect of Mg.
Is there significance to the fact that this disease is most common in children, and thus less likely to be influenced by changes induced by old age? Pre-treatment hypomagnesemia has been reported in young leukemic children, 78% of whom have histories of anorexia, and have excessive gut and urinary losses of Mg.(195) In addition, strains of rats that develop lymphosarcoma and/or leukemia (on very high Ca/Mg intakes), or that exhibit thymic atrophy when fed Mg deficient diets with less unbalanced Ca/Mg ratios,(98) do so only when deficiency is induced at weaning or a short time thereafter.(10-12) That infantile hypomagnesemia, that may be worsened by calcemic treatment of the secondary hypocalcemia that is usually detected first, can be associated with evidence of pathologic Ca excess (i.e as renal tubular calcinosis.)(40,196,197)
The prevalence of low Mg intakes (below 300 mg/day) in the American population,(39-45) has special significance among pregnant women,(198,199) who may be advised to increase their Ca intake to as much as 2 grams/day.(200) In view of their low Mg intake, this would bring the Ca/Mg ratio of pregnant women to 6/1. Such a Mg deficit of the mother is likely to be reflected by at least as great a deficit in the infant, that could result in development of infantile hypomagnesemic hypocalcemia.(40,198) Analysis of metabolic balance studies showed that a 2/1 ratio of dietary Ca/Mg maintained balances for both, with Mg intakes of over 300-400 mg/day.(39) A 4/1 ratio is realized with the 1,200 mg suggested daily intake of Ca for prophylaxis against osteoporosis.(201) Might the relative Mg deficiency induced by these widely recommended Ca intakes, without simultaneously increasing the intake of Mg, increase the risk, not only of cardiovascular complications,(202) but of initiation of neoplastic transformation of cells?
Are rat-strain differences in susceptibility to Mg deficiency-induced lympholeukemia related to genetic differences in Mg retention and tissue levels, as has been shown for mice?(203) Human genetic errors of Mg metabolism are known: intestinal Mg malabsorption(195,196,204-207) and renal Mg wastage.(208-213) Not reported is whether families with these metabolic errors have increased vulnerability to malignancies. Abnormal (high) erythrocyte Mg levels have been found in members of families with leukemia,(214) such as has been reported in other neoplastic patients in active phases of their malignancies, that accompany the rise in tumor Mg levels during its active phase.(13,97,215,216) An HLA-associated genetic factor has been shown to play a role in control of Mg levels.(217-219)
Not considered earlier is the possibility that low serum Mg/Ca levels might be a factor in metastatasis. There is evidence that it is contributory to hypercoagulability;(202) possibly, this might participate in neoplastic cell adhesiveness and spread.(215,216) Might deficiency of Mg and/or Zn during pregnancy(40,135,196,199) impair the lymphopoietic system of the fetus? Might infantile deficiencies of one or more of the nutrients: Mg, Zn and B6 also predispose to immunologic deficits(135,198) that might be a factor in vulnerability to early and later malignancies?
Despite provocative findings that suggest that Mg deficiency might be implicated in aspects of pathogenesis and treatment of neoplasms, there are many unknowns. Investigation of these questions might lead to means to prevent lympholeukemias, or possibly of immuno-incompetence. Whether higher Mg intakes might be protective against oncogens in humans as it is in some animal models deserves study.
REFERENCES
1. Seelig, M.S. Magnesium (and trace substance) deficiencies in the pathogenesis of cancer. Biol. Tr. Elem. Res. 1979; 1:273-297.
2. Wacker, W.E.C. Magnesium and Man. Harvard Univ. Press, Cambridge, MA, 1980.
3. Aikawa, J.K. Magnesium: Its Biologic Significance. CRC Press, Boca Raton, FL, 1981.
4. Heaton, F.W., Rayssiguier, Y. Magnesium deficiency and membrane properties. in Magnesium in Cellular Processes and Medicine. Altura, B.M., Durlach, J. and Seelig, M.S. (from 4th Intl Mg Sympos, Blacksburg, W. Virginia, 1985), S. Karger AG, Basel, Switzerland, 1987; 121-130.
5. Heaton, F.W., Tongyai, S., Rayssiguier, Y. Membrane function in magnesium deficiency. in Magnesium in Health and Disease. (from 5th Intl Mg Sympos, Kyoto, Japan, 1988) Itokawa, Y. and Durlach J., Eds., John Libbey Press, London, Paris, 1989; 27-33.
6. Guenther, T., Averdunk, R. Membranes of magnesium deficiency induced neoplastic cells. Magnesium Bull. 1985; 7:146-151.
7. Walker, G.M. Magnesium as the fundamental regulator of the cell cycle. Magnesium 1982; 2:1-16.
8. Walker, G.M. Magnesium and cell cycle control: an update. Magnesium 1986; 5:9-23.
9. Duffus, J.H., Walker, G.M. Magnesium in mitosis and the cell cycle. In Magnesium in Cellular Processes and Medicine, (from 4th Intl Mg Sympos, Blacksburg, W. Virginia, 1985 Eds B.M. Altura, J. Durlach, M.S. Seelig, Publ. S. Karger AG, Basel, Switzerland, 1987; 131-141.
10. Hass, G.M., Laing, G.H., Galt, R.M., McCreary, P.A. Recent advances: immunopathology of magnesium deficiency in rats: induction of tumors; incidence, transmission and prevention of lymphoma-leukemia. Magnesium Bull. 1981; 3:217-228.
11. Hass, G.M., Laing, G.H., Galt, R.M., McCreary, P.A. Role of magnesium deficiency in immunity to neoplasia in the rat. Magnesium Bull. 1981; 3:5-11.
12. Hass, G.M., McCreary, P.A., Laing, G.H., Galt, R.M. Lymphoproliferative and immumunologic aspects of magnesium deficiency. In Magnesium in Health and Disease (from 2nd Intl Mg Sympos, Montreal, Canada, 1976), b Eds. M. Cantin, M.S. Seelig, Publ. Spectrum Press, NY, 1980, pp 185-200.
13. Collery, P., Anghileri, L.J., Coudoux, P., Durlach, J. (Magnesium and cancer: Clinical data.) Magnesium Bull. 1981; 3:11-20.
14. Bazikian, K.L. The significance of magnesium salts in oncology. Proc of First Intl Mg Sympos, Vittel, France, Ed. J. Durlach (private printer) 1971; 593-606.
15. Aleksandrowicz, J. Natural environment and health. in Protection of Man's Natural Environment Polish Sci. Publ. 1973; 518-528.
16. Aleksandrowicz, J., Blicharski, J., Dzigowska, A., Lisiewicz, J. Leuko- and oncogenesis in the light of studies on metabolism of magnesium and its turnover in biocenosis. Acta Med. Pol. 1970; 11:289-302. (abstr: Blood 1971; 37:245)
17. Aleksandrowicz, J. (Mycotoxins, bioelements, and perspectives in prophylaxis in the ecology of leukemia). Rev. Esp. Encologia 1975; 22:311-333.
18. Aleksandrowicz,J., Skotnicki, A.B., (transl by E. Nowak) Leukemia Ecology. Ecological Prophylaxis of Leukemia. (Avail from U.S. Dept. Commerce, Natl. Techn. Inform. Serv. Springfield VA 22161) 1982.
19. Hopps, H.C. What causes senescence? In Aging and the Geochemical Environment, Report of Panel, Natl. Res. Council, N.A.S., Natl. Acad. Press, Washington D.C. 1981; 42-71.
20. Hopps, H.C. How might geochemical factors affect senescence and age-associated pathology? In Aging and the Geochemical Environment. Report of Panel, Natl. Res. Council, N.A.S., Natl. Acad. Press, Washington D.C. 1981; 118-126.
21. Jasmin, G. (Lymphedema, hyperplasia, tumefaction of lymphatic tissue of the rat kept on a magnesium deficient diet). Rev. Can. Biol. 1963; 22:383-390.
22. Bois, P. Tumour of the thymus in magnesium-deficient rat. Nature 1964; 204:1316.
23. Bois, P. Peripheral vasodilatation and thymic tumors in magnesium deficient rats. In Endocrine Aspects of Disease Processes. Ed. G. Jasmin, Publ. W.H. Greene Inc, St, Louis, Missouri, USA 1968; 337-355.
24. Bois, P., Sandborn, E.B., Messier, P.E. A study of thymic lymphosarcoma developing in magnesium-deficient rats. Cancer Res. 1976; 29:763-775.
25. McCreary, P.A., Battifora, H.A., Hahneman, B.M., Laing, G.H., Hass, G.M. Leukocytosis, bone marrow hyperplasia and leukemia in chronic magnesium deficiency in the rat. Blood 1967; 29:683-690.
26. Battifora, H.A., McCreary, P.A., Hahneman, B.M., Laing, G.H. Chronic magnesium deficiency in the rat. Studies of chronic myelogenous leukemia. Arch. Path. 1968; 122:610-620.
27. Battifora, H.A., McCreary, P.A., Laing, G.H., Hass, G.M. Chronic granulocytic leukemia and malignant lymphoma in magnesium deficient rats. Am. J. Path. 1969; 55:11a.
28. Battifora, H. Effects of magnesium deficiency on blood cells. Clinical and experimental data. In Proc First Intl Mg Sympos, Vittel, France Ed. J. Durlach (private printer) 1971:501-516.
29. McCreary, P.A., Laing, G.H., Hass, G.M. Susceptibility of normal and magnesium deficient rats to weekly subtumorigenic doses of live lymphoma cells. Am. J. Path. 1973; 7:89.
30. Hunt, B.J., Belanger, L.F. Localized, multiform, sub-periosteal hyperplasia and generalized osteomyelosclerosis in magnesium-deficient rats. Calcif. Tiss. Res. 1972; 9:17-27.
31. Belanger, L.F. Medullary bone and periosteal tumour formation in magnesium deficient rats. In Proc First Intl Mg Sympos, 1971, Vittel, France Ed. J. Durlach (private printer) 1973; 2:425-430.
32. Belanger, L.F., Hunt, B.J., Narbaitz, R. Alkaline phosphatase in hyperplastic bone. Lesions of rats fed a magnesium deficient diet. In Histochemistry and Cytochemistry, Eds T. Takeuchi, K. Ogawa, S. Fujita Publ. Nakashani, Kyoto, Japan 1972; 313-314.
33. Bogoroch, R., Belanger, L.F. Skeletal effects of magnesium deficiency in normal, ovariectomized, and estrogen-treated rats. Anat. Rec. 1975; 183:437-448.
34. Hopps, H.C. Geochemical Environment and Cancer. Geochemistry and the environment. III Distribution of trace elements related to the occurrence of certain cancers, cardiovascular diseases, and urolithiasis. Proc. Workshop, Captiva Island, FL, 1974. Natl. Res. Council, N.A.S. 1977.
35. Sauer, H.I. The definition and demographic characterization of an increased longevity area in the United States. In Aging and the Geochemical Environment. Natl. Res. Council, N.A.S., Natl. Acad. Press, Washington, D.C. 1981; 10-17.
36. Schroeder, H.A. Relations between hardness of water and death rates from certain chronic and degenerative disease in the United States. J. Chron. Dis. 1960; 12:586-591.
37. Anderson, T.W., Neri, L., Schreiber, G.B., Talbot, F., Zdrowjewski, A. Ischemic heart disease, water hardness and myocardial magnesium. Can. Med. Assoc. J. 1975; 113:199-203.
38. Marier, J.R. Cardioprotective contribution of hard water to magnesium intake. Rev. Can. Biol. 1978; 37:115-125.
39. Seelig, M.S. The requirement of magnesium by the normal adult. Am. J. Clin. Nutr. 1964; 14:342-390.
40. Seelig, M.S. Magnesium Deficiency in the Pathogenesis of Disease. Early Roots of Cardiovascular, Skeletal, and Renal Abnormalities. Ed. L.V. Avioli, Publ. Plenum Med. Book Co., New York, N.Y., 1980.
41. U.S. Dept. Agric. Nationwide Food Consumption Survey: Food and nutrient intakes of individuals in 1 day in the U.S.A., 1977-1978, 1980.
42. Pao EM, Mickle SJ: Problem Nutrients in the United States. Food Technol. 35:58-69, y1981.
43. Morgan, K.J., Stampley, G.L., Zabik, M.E., Fischer, : Magnesium and calcium dietary intakes of the U.S. population. J. Am. Coll. Nutr. 1985; 4:195-206.
44. Morgan, K.J., Stampley, G.L. Dietary intake levels and food sources of magnesium and calcium for selected segments of the US population. Magnesium 1988; 7:225-233.
45. Seelig, M.S. Human requirements of magnesium; factors that increase needs. In Proc. 1st Intl Mg Sympos, Vittel, France, Ed. J. Durlach; (private printer) 1971; 10-28.
46. Seelig, M.S., Heggtveit, H.A. Magnesium interrelationships in ischemic heart disease. Am. J. Clin. Nutr. 1974; 27:59-79.
47. Seelig, M.S. Magnesium requirements in human nutrition. (from 3rd Intl. Mg Sympos., 1981, Baden-Baden, Germany) Magnesium Bull.1981; 3(1a):26-47.
48. Seelig, M.S. Nutritional status and requirements of magnesium, with consideration of individual differences and prevention of cardiovascular disease. (from 2nd Europ. Congr. on Mg, Stockholm, Sweden, 1986), Magnesium Bull. 1986; 8:170-185.
49. Seelig, M.S. Cardiovascular consequences of magnesium deficiency and loss: pathogenesis, prevalence and manifestations - magnesium and chloride loss in refractory potassium repletion. Am. J. Cardiol. 1989; 63:4G-21G.
50. Altura, B.M., Altura, B.T. New perspectives on the role of magnesium in the pathophysiology of the cardiovascular system. Clinical aspects. Magnesium 1985; 4:226-244.
51. Anderson, T.W., LeRiche, W.H. Sudden death from ischemic heart disease in Ontario and its correlation with water hardness and other factors. Can. Med. Assoc. J. 1971; 105:155-160.
52. Anderson, T.W., Leriche, W.H., Hewitt, D., Neri, L.C.: Magnesium, water hardness, and heart disease. In Magnesium in Health and Disease, (from 2nd Intl. Mg Sympos., Montreal, Canada, 1976), Eds., M. Cantin, M.S. Seelig, Publ. Spectrum Press, NY, 1980; 565-571.
53. Neri, L.C., Johansen, H.L., Hewitt, D., Marier, J., Langner, N. Magnesium and certain other elements and cardiovascular disease. Sci. Tot. Environm. 1985; 42:49-75.
54. Karppannen, H. Epidemiologic evidence for considering magnesium deficiency as a risk factor for cardiovascular diseases. Magnesium Bull. 1990; 12:80-86.
55. Burnet, F.M. An immunologic approach to aging. Lancet 1970; 2:358-370.
56. Greenberg, L.J., Yunic,E.Y. Genetic control of autoimmune disease and immune responsiveness and the relationship to aging. Birth Defects. Orig Article Ser 1978; 14:249-260.
57. Makinodan, Y., Kay, M.M.B. Age influence on the immune system. Adv. Immunol. 1980; 29:287-330.
58. Haury, V.G. Variations in serum magnesium in health and disease: A review. J. Lab. Clin. Med. 1942; 27:1361-1375.
59. Delbet, P. Presse Med. 1928; 36:1473. (cited by Haury #58)
60. Delbet, P., Robinet, L. Terrains magnesiens et cancer. Bull. Acad. Med. Paris 1932; 111:415. (cited by Haury #58)
61. Bablet, J., Bader, H. Magnesium and cancer in Indochina. Bull. Assoc. Franc.l'Etude de Cancer 1932; 21:570. (cited by Haury #58)
62. Schrupf-Peirron, P. (Causes of rarity of cancer in Egypt). Bull. Acad. Med. Paris 1931; 105:818, 106:235. (cited by Haury, #58)
63. Marchi, C. Carenza di cloruro di magnesio e maggior frequenza di canril not a preventiva. Seritta Biol. 1930, 4:295. (cited by Haury, #58)
64. Heinemann, H. Hippokrates 1953; 24:744. (cited by Bazikian, #14)
65. Neiper, H.A. Med. Welt 1953; 154:272, 1282. (cited by #14)
66. Akemtsev, V. Pochvovedie 1957; 7:91, 1957 (cited by Bazikian, #14)
67. Akemtsev, V., Milin, Z., Smolianionov, J. Moskva 1966; 45:1966. (cited by Bazikian, #14)
68. Balasanian, S. Geokhimia 1959; 3:226, 1959. (cited by Bazikian #14)
69. Miron, W., Sobaniec-Lotowska, M., Sulkowski, S. [Malignant neoplasms in autopsy specimens and the magnesium level in the soil of the communities of Grodek and Tykocin.] Wiad. Lek. 1989; 42:1033-1074.
70. Armstrong, R.W. Is there a particular kind of soil or geologic environment that predisposes to cancer? Ann. N.Y. Acad. Sci. 1972; 199:239-248.
71. World Health Organization Report: Prevention of cancer Tech Rep Ser 276, 1964) & Intl Agency on Cancer, Lyons 1977 (cited by Aleksandrowicz, #18)
72. Mason, T.J., MacKay, F.W., Blot, W.J., Fraumeni, J.F. Jr. Atlas of Cancer Mortality for U.S. Counties: 1950-1969. 1975; DHEW Publ #NIH 75-780.
73. Feder, G.L., Hopps, H.C. Variations in drinking water quality and the possible effects on human health. Tr. Subst. Envirnm. Hlth. 1981; 15:96-103.
74. Kashiwa, H.K., Hungerford, G.F. Blood leucocyte response in rats fed a magnesium deficient diet. Proc. Soc. Exp. Biol. Med. 1958; 99:441-443.
75. Hungerford, G..F, Karson, E.F. The eosinophilia of magnesium deficiency. Blood 1960; 16:1642-1650.
76. Guenther, T., Vormann, J., Hoellrieg, V., Disch, G., Classen, H.G. Role of lipid peroxidation and vitamin E in magnesium deficiency. J. Nutr. Biochem. 1993; (in press)
77. Belanger, L.F., Medullary bone and periosteal tumour formation in magnesium deficient rats. in Proc. First Intl. Mg Sympos. Vittel, France 1971 Ed. J.Durlach, (private printer) 1973; 2:425-430.
78. Belanger, L.F., Hunt, B.J., Narbaitz, R. Alkaline phosphatase in hyperplastic bone. Lesions of rats fed a magnesium deficient diet. In Histochemistry and Cytochemistry, Eds Takeuchi T, Ogawa K, Fujita S, Publ. Nakashani, Kyoto, Japan, 1972; 313-314.
79. Hunt, B.J., Belanger, L.F. Localized, multiform, sub-periosteal hyperplasia and generalized osteomyelosclerosis in magnesium-deficient rats. Calcif. Tiss. Res. 1972; 9:17-27.
80. Belanger, L.F., Robichon, J. The effects of magnesium deficiency on the host response to intramuscular bone matrix implanted in the rat. J. Bone Joint Surg. 1975; 57A:522-526.
81. Lai, C.C., Singer, L., Armstrong, W.D. Bone composition and phosphatase activity in magnesium deficiency in rats. J. Bone Joint Surg. 1975; 57:516-522.
82. Vormann, J., Mercker, H.J., Barrach, H.J., Stolpmann, H.J., Averdunk, R., Guenther, T. Induction of a tumour-like proliferation in the intestine of magnesium deficient rats. Magnesium Bull. 1985; 7:4-10.
83. Delbet, P., Palios, C. (Halogen salts of magnesium and experimental cancer). Bull. Acad. Med. Paris 1931; 105:508. (cited by Haury, 59)
84. Sugiura, K., Benedict, S.R. Influence of magnesium on the growth of carcinoma, sarcoma and melanoma in animals. Am. J. Cancer 1935; 23:300- 310.
85. Mills, B.J., Broghamer, B.L., Higgins, P.J., Lindeman, R.D. Inhibition of tumor growth by magnesium depletion of rats. J. Nutr. 1984; 114:739-745.
86. Yan, L., Boylan, L.M., Spallholz, J.E. Effect of dietary selenium and magnesium on human mammary tumor growth in athymic nude mice. Nutr. Cancer 1991; 16:239-248.
87. Shilovtsev, S., Shilovtseva, A. Skolnikova, S., Krichevsky, A. The prophylactic and curative effects of magnesium salts, calcium and bromides in malignant tumors. Materials of Kwibishev Med Inst 1963; 11 (cited by Bazikian, #14)
88. Gharibjian, B., Zakharian, R. Action of magnesium ions on the toxicity and antiblastomogenic activity of thiophosphamide. Rep. Armenian Acad. Sci. 30 (cited by Bazikian, #14)
89. Mori, H., Morishita, Y., Mori, Y., Yoshimi, N., Sugie, S., Tanaka, T. Effect of magnesium hydroxide on methylazoxymethanol acetate-induced epithelial proliferation in the large bowels of rats. Cancer Lett. 62:43-48, 1992.
90. Poirier, L.A., Theiss, J.C., Arnold, L.J., Shimkin, M.B. Inhibition by magnesium and calcium acetates of lead subacetate- and nickel acetate- induced lung tumors in strain A mice. Cancer Res. 1984; 44:1520-1522.
91. Kasprzak, K.S., Quander, R.V., Poirier, L.A. Effects of calcium and magnesium salts on nickel bisulfide carcinogenicity in Fischer rats. Carcinogenesis 1985; 6:1161-1166.
92. Kasprzak, K.S.,Ward, J.M., Poirier, L.A., Reichardt, D.A., Denn, A.C., Reynolds, C.W. Nickel-magnesium interactions in carcinogenesis; dose effects and involvement of natural killer cells. Carcinogenesis 1987; 7:1005-1011.
93. Kasprzak, K.S., Kiser, R.F., Weislow, O.S. Magnesium counteracts nickel-induced suppression of T-lymphocyte response to concavalin A. Magnesium 1988; 7:166-172.
94. Smialowicz, R., Rogers, R.R., Riddle, M.M., Luebke, R.W., Fogelson, L.D., Rowe, D.G. Effects of manganese, calcium, magnesium and zinc on nickel- induced suppression of natural killer cell activity. J. Toxicol. Environm. Hlth. 1987; 20:67-80.
95. Conway, K., Wang, S.W., Xu, L.S., Costa, M. Effect of magnesium on nickel-induced genotoxicity and cell transformation. Carcinogenesis 1988; 8:1115-1121.
96. Poirier, L.A., Kazprzak, K.S., Hoover, K.L., Wenk, M.L. Effects of calcium and magnesium acetates on the carcinogenicity of cadmium chloride in Wistar rats. Cancer Res. 1983; 43:4575-4581.
97. Durlach, J., Bara, M., Guiet-Bara, A., Collery, P. Relationship between magnesium, cancer and carcinogenic or anticancer metals. Anticancer Res. 1986; 6:1353-1361.
98. Alcock, N.W., Shils, M.E., Lieberman, P.H., Erlandson, R.A. Thymic changes in the magnesium depleted rat. Cancer Res. 1973; 33:2196-2204.
99. Whitfield, J.F., Perris, A.D., Youdale, T. The calcium mediated promotion of mitotic activity in rat thymocyte populations by growth hormone, neurohormones, parathyroid hormone, and prolactin. J. Cell Physiol. 1969; 73:203-212.
100. Whitney, R.B., Sutherland, R.M. The influence of calcium, magnesium and cyclic adenosine 3'5'-monophosphate on the mixed lymphocyte reaction. J. Immunol. 1972; 108:1179-1183.
101. Abboud, C.N., Scully, S.P., Lichtman, A.H., Brennan, J.K., Segel, G.B. The requirements for ionized calcium and magnesium in lymphocyte proliferation. J. Cell. Physiol. 1985; 122:64-72.
102. Brennan, J.K., Seelig, C.B., Lichtman, M.A. The role of magnesium in neutrophil production and function. In Magnesium in Health and Disease (from 2nd Intl Mg Sympos, Montreal, Canada, May, 1976), Eds. M. Cantin, M.S. Seelig, Publ. Spectrum Press, NY, 1980, pp 169-183.
103. Brennan, J.K., Lichtman, M.A., Chamberlain, J.K., Leblond, P. Isolation of variant lymphoma cells with reduced growth requirements for extracellular calcium and magnesium and enhanced oncogenicity. Blood 1976; 47:447-469.
104. Guenther, T., Averdunk, R., Wonigeit, K., Vormann, J. Characterization and growth factor production of magnesium deficiency-induced malignant T cell lymphomas. Magnesium Bull. 1988; 10:22-26.
105. Maguire M.E., Magnesium and cell proliferation. Ann. N.Y. Acad. Sci. 1988; 551:201-217.
106. Rubin, H. Central Role for Magnesium in Coordinate Control of Metabolism and Growth in Animal Cells. Proc. Natl. Acad. Sci. USA 1975; 72:3551- 3555.
107. Rubin, H., Koide, T. Mutual potentiation by magnesium and calcium of growth in animal cells. Proc. Natl. Acad. Sci. USA 1976; 73:168-172.
108. Rubin H: Differences in growth requirement and retentiveness for magnesium in nontransformed and transformed mouse 3T3 cells. Magnesium 1:41-48, 1982.
109. Morgan, J.I., Perris, A.D. The influence of sex steroids on calcium- and magnesium-induced mitogenesis in isolated rat thymic lymphocytes. J. Cell. Physiol. 1974; 83:287-296.
110. Okazaki T., Yumoto Y., Okuda T., Kato Y., Tashima M., Sawada H., Uchino H. Magnesium deprivation inhibits the expression of differentiation-related phenotypes in HL-60 Cells. Magnesium Res. 1988; 1:114. 111. Cittadini, A., Wolf,, F.I., Bossi, D., Calviello, G. Magnesium in normal and neoplastic cell proliferation: state of the art on in vitro data. Magnesium Res. 1991;4:23-33.
112. Grubbs, R.D., Maguire, M.E. Magnesium as a Regulatory Cation: Criteria and Evaluation. Magnesium 1987; 6:113-127.
113. Guenther, T. Functional compartmentation of intracellular magnesium. Magnesium 1986; 5:53-59.
114. Elin, R.J., Armstrong, W.D., Singer, L. Enzyme, adenosine triphosphate, and blood cellular changes in magnesium deficient and control rats. Proc. Soc. Exp. Biol. Med. 1971; 137:635-640.
115. Rayssiguier, Y. Lipoprotein metabolism; importance of magnesium. Magnesium 1986; 186-193.
116. Guenther, T., Vormann, J., Merker, H.J., Averdunk, R., Peter, H.W., Wonigeit, K. Membrane alterations in magnesium-deficiency-induced malignant T Cell lymphoma. Magnesium 1984; 3:29-37.
117. Averdunk, R., Guenther, T. Phospholipid metabolism and concanavalin A stimulation of thymocytes from magnesium-deficient rats and magnesium deficiency-induced T-cell lymphoma. Magnesium Bull. 1985; 7:11-15.
118. Gossrau, R., Vormann, J., Guenther, T. Enzyme Histochemistry of Malignant T Cell Lymphoma due to Chronic Magnesium Deficiency in Rats. Histochem. 1984; 80:183-186.
119. Anghileri, L.J. Magnesium concentration variations during carcinogenesis. Magnesium Bull. 1979; 1:46-48.
120. Anghileri, L.J., Collery, P., Coudoux, P., Durlach, J. (Experimental relationships between magnesium and cancer.) Magnesium Bull. 1981; 3:1-5. 121. Anghileri, L.J., Heidbreder, M., Weiler, G., Dermietzel, R. Hepatocarcinogenesis by thioacetamide: correlations of histological and biochemical changes, and possible role of cell injury. Exp. Cell. Biol. 1977; 45:34-47.
122. Blondell, J.W. The anticancer effect of magnesium. Medical Hypothesis 1980; 6:863-871.
123. Petitou, M., Tuy, F., Rosenfeld, C., Mishal, Z., Paintrand, M., Jasmin, C., Mathe, G., Inbar, M. Decreased microviscosity of membrane lipids in leukemic cells; two possible mechanisms. Proc. Natl. Acad. Sci. USA 1978; 75:2306-2310.
124. Mulay, I.L., Roy, R., Knox, B.E., Suhr, N.H., Delaney, W.E. Trace-Metal Analysis of cancerous and Non-Cancerous Human Tissues. J. Natl. Cancer Inst. 1971; 47:1-13.
125. Anghileri, L.J., Miller, E.S., Robinette, J., Prasad, K.N., Lagerborg, V.A. Calcium metabolism in tumors. II. Calcium, magnesium and phosphorus in human and animal tumors. Oncology 1971; 25:193-209.
126. Digiesi, V., Bandinelli, R., Bisceglie, P., Santoro, E. Magnesium in tumoral tissues, in the muscle and serum of subjects suffering from neoplasia. Biochem. Med. 1983; 29:360-363.
127. Szmeja, Z., Koenczewska, H. Red blood cell, serum and tissue magnesium levels in subjects with laryngeal carcinoma. J. Otorhinolaryngol. Relat. Spec. 1983; 45:102-107.
128. Ranade, S.S., Panday, V.K. Major metals in human cancer: calcium, magnesium, sodium and potassium. Sci. Total Environm. 1985; 41:79-89.
129. Taylor, J.S., Vigneron, D.B., Murphy-Boesch, J., Nelson, S.J., Kessler, H.B., Coia, L., Curran, W., Brown, T.R. Free magnesium levels in normal human brain and brain tumors: 31P chemical-shift imaging measurements at 1.5 T. Proc. Natl. Acad. Sci. USA 1991; 88:6810-6814.
130. Chandra, R.K., Newberne, P.H. 5Nutrition, Immunity and Infection. Plenum Press, New York, 1977.
131. McCoy, J.H., Kenney, M.A. Magnesium and immune function: a review. In Magnesium in Cellular Processes and Medicine. Eds: B.M. Altura, J. Durlach, M.S. Seelig, Publ. S. Karger, Basel, Switzerland 1987: 196-211.
132. Flynn, A., Loftus, M.A., Finke, J.H. Production of interleukin-1 and interleukin-2 in allogenic mixed lymphocyte cultures under copper, magnesium and zinc deficiency. Nutr. Res. 1984;4:673-679.
133. Flynn, A., Yen, B.R. Mineral deficiency effects on the generation of cytotoxic T-cells and T-helper cell factors in vitro. J. Nutr. 1981; 111:907-913.
134. Durlach, J. (Mechanisms of synergy between vitamin B6 and magnesium.) J. Med. Besancon 1969; 349-359.
135. Seelig MS: Nutritional roots of combined system disorders. In Clinical Disorders in Pediatric Nutrition. Ed. F. Lifshitz, Publ. Marcel Dekker, New York, N.Y.1982; 327-351.
136. Seelig, M.S. Possible role of magnesium in disorders of the aged. In Intervention in the Aging Process. A: Quantitation, Epidemiology, Clinical Research. Eds W. Regelson, F.M. Sinex, Publ A. Liss, New York, N.Y.1983; 279-305.
137. Durlach, J. Magnesium in Clinical Practice. (transl. by D. Wilson), Publ. John Libbey & Co, London, U.K. 1988.
138. Evans, G.W. Normal and abnormal zinc absorption in man and animals; the tryptophan connection. Nutr. Rev. 1980; 38:137-141.
139. Hsu, J.M. Zinc content in tissues of pyridoxine deficient rats. Proc. Soc. Exp. Biol. Med. 1965; 119:177-180.
140. Chandra, R.K., Au, B. Single nutrient deficiency and cell-mediated immune response. Pyridoxine. Nutr. Res. 1981; 1:101-106.
141. Beisel, W.R. Single nutrients and immunity. Am. J. Clin. Nutr. 1982; 417-468.
142. Bendich, A., Cohen, M. B vitamins: effects on specific and nonspecific immune responses. In Nutrition and Immunology Ed. R.J. Chandra, Publ. A.R. Liss, Inc. New York, N.Y. 1988; 101-123.
143. Greenberg, L.D., Bohr, D.F., McGrath, H., Rinehart, J.F. Xanthurenic acid excretion in the human subject on a pyridoxine-deficient diet. Arch. Biochem. 1949; 21:237-239.
144. Wachstein, M., Lobel, S. The relation between tryptophane metabolism and vitamin B6 in various diseases as studied by paper chromatography. Am. J. Clin. Path. 1956; 26:910-925.
145. Rose, D.P. Tryptophan metabolism in carcinoma of the breast. Lancet 1967; 1:239-241.
146. Yoshida, O., Brown, R.R., Bryan, G.T. Relationship between tryptophan metabolism and heterotopic recurrences of human urinary bladder tumors. Cancer 1970; 25:773-780.
147. Brown, R.R., Friedell, G.H., Leklem, J.E. Tryptophan metabolism in patients with bladder cancer. Am. Indust. Hyg. Assoc. 1972; 33:217-222.
148. Davis, H.L., Brown, R.R., Leklem, J., Carlson, I.H. Tryptophan metabolism in breast cancer. Cancer 1973; 31:1061-1064.
149. Gailani, S., Murphy, G., Kenny, G., Nussbaum, A. Silvernail, P. Studies on tryptophan metabolism in patients with bladder cancer. Cancer Res. 1973; 33:1071-1076.
150. Leklem, J.E., Brown, R.R. Abnormal tryptophan metabolism in a family with a history of bladder cancer. J. Natl. Cancer Inst. 1976; 1101-1104.
151. Leklem, J.E., Brown, R.R., Potera, C., Becker, D.S. A role for vitamin B6 in Cancer. In Nutrition and Cancer. Eds. J. VanEys, M.S. Seelig, B.L. Nichols, publ.SP Medical Books, New York, 1979: 249-261.
152. Bryan, G.T., Brown, R.R., Price, J.M. Mouse bladder carcinogenicity of certain tryptophan metabolites and other aromatic nitrogen compounds suspended in cholesterol. Cancer Res. 1964; 24:596-602.
153. Moynahan, E.J. Zinc deficiency and cellular immune deficiency in acrodermatitis enteropathica in man and zinc deficiency with thymic hypoplasia in Fresiam calves: a possible genetic link. Lancet 1975; 2:710.
154. Weston, W.L., Huff, J.C., Humbert, J.R. Zinc correction of defective chemotaxis in acrodermatitis enteropathica. Arch. Dermatol. 1977; 113: 422-425.
155. Fernandes, G,., Nair, M., Kazunori, O., Tanaka, T., Floyd, R., Good, R.A. Impairment of cell-mediated immunity functions by dietary zinc deficiency in mice. Proc. Natl. Acad. Sci. USA 1979; 76:457-461.
156. Gross, R.L., Osdin, N., Fong,L., Newberne, P.M. Depressed immunologic function in zinc deprived rats as measured by mitogen response of spleen, thymus and peripheral blood. Am. J. Clin. Nutr. 1979; 32:1260-1266.
157. Pekarek, R.S., Sandstead, H.H., Jacob, R.A., Barcome, D.F. Abnormal cellular immune responses during acquired zinc Deficiency. Am. J. Clin. Nutr. 1979; 32:1466-1471.
158. Cunningham-Rundles, S., Cunningham-Rundles, W. Zinc modulation of immune response. In Nutrition and Immunology. Ed. R.J. Chandra, Publ. A.R. Liss, Inc. New York, N.Y. 1988; 197-214.
159. Pories, W.J., Mansour, E.G., Strain, W.H. Trace elements that act to inhibit neoplastic growth. Ann. N.Y. Acad. Sci. 1972; 199:265-274.
160. Kasprzak,K.S., Waalkes, M.P. The role of calcium, magnesium, and zinc in carcinogenesis. Adv. Exp. Med. Biol. 1986; 206:497-515.
161. Harman, D., Heidrick, M.L., Eddy, D.E. Free radical theory of aging; effect of free radical reaction inhibitors on the immune response. J. Am. Geriatr. Soc. 1977; 25:400-407.
162. Leibovitz, B.E., Siegel, B.V. Aspects of free radical reactions in biological systems. J. Gerontol. 1980. 35:45-56.
163. Menkes, M.S., Comstock, G.W., Vuilleumier, J.P., Helsing, K.J., Rider, A.A., Brookmeyer R. Serum Beta-Carotene, Vitamins A and E, Selenium, and the Risk of Lung Cancer. New Engl. J. Med. 1986; 315:1250-1254.
164. Goldsmith, L.A. Relative magnesium deficiency in the rat. J. Nutr. 1967; 93:87-102.
165. Blaxter, K.L., Wood, W.A. The nutrition of the young ayreshire calf. 9. Composition of the tissues of normal and dystrophic calves. Brit. J. Nutr. 1952; 6:144-163.
166. Schwarz, K. Vitamin E, trace elements, and sulfhydryl groups in respiratory decline. Vitamins Hormones. 1962; 20:463-484.
167. Zuckerman, L. Marquardt, G.H. Muscle, erythrocyte, and plasma electrolytes and other muscle constituents of rabbits with nutritional muscular dystrophy. Proc. Soc. Exp. Biol. Med. 1963; 112:609-610.
168. Tansy, M.F. Intestinal absorption of magnesium. In Intestinal Absorption of Metal Ions, Trace Elements, and Radionuclides. Eds. S.C. Skoryna, D. Waldron-Edwards. Publ. Pergamon Press, New York 1970; 193-210.
169. Blount, H.C.: Effect of magnesium on the response of mice to large doses of whole-body irradiation. Radiology 1955; 65:250-253.
170. Tansy, M.F., Nichini, F.M., Baker, H.W., Chrzyanowski, J. Association of low serum magnesium concentration with severity of gastrointestinal symptomatology in the irradiated patient. J. Surg. Res. 1971; 11:213-216. 171. Nichini, F.M., Tansy, M.F., Kendall, F.M. Serum magnesium fall in radiation-induced gastrointestinal symptomatology. Radiology 1973; 108:413-415.
172. Evard, D., Sebag, A., Cosnes, J., Gendre, J.P. (Hypomagnesemia in enteritis from chronic irradiation). Med. Chir. Dig. 1983; 12:509-513.
173. Cohen, L., Kitzes, R. Early radiation-induced proctosigmoiditis responds to magnesium therapy. Magnesium 1985; 4:16-19.
174. Bell, D.R., Woods, R.L., Levi, J.A. Cis-diaminedichloroplatinum-induced hypomagnesemia and renal magnesium wasting. Europ. J. Cancer Clin. Oncol. 1985; 21:287-290.
175. Vogelsang, N.J., Torkelson, Kennedy, B.J. Hypomagnesemia, renal dysfunction, and Raynaud's phenomenon in patients treated wth cisplatin, vinblastine and bleomycin. Cancer 1985; 56:2765-2770.
176. Mavichak, V., Coppin, C.M., Wong, N.L., Dirks, J.H., Walker, V., Sutton R.A. Renal magnesium wasting and hypocalciuria in chronic cis-platinum nephropathy in man. Clin. Sci. 1988; 75:203-207.
177. Daugaard, G., Abildgaard, U., Holstein-Rathlou, N.H., Bruunshuus, I., Bucher, D., Leyssac, P.P. Renal tubular function in patients treated with high-dose cisplatin. Clin. Pharmacol. Ther. 1988; 44:164-172.
178. DeLeeuw, I., De Beukelaer, T., Hartoko, T., Van Gaal, L., Vandewoude, M. Effects of cisplatinum and cyclosporin on magnesium status in clinical oncology. Magnesium Res. 1988. 1:115, 1988.
179. Markmann, M., Rothman, R., Reichman, B., Hakes, T., Lewis, J.L. Jr., Rubin, S., Jones, W., Almadrones, L., Hoskins, W. Persistent hypomagnesemia following cisplatin chemotherapy in patients with ovarian cancer. J. Cancer Res. Clin. Oncol. 1991; 117:89-90.
180. Abbasciano, V., Mazzotta, D., Vecchiatti, G., Tassinari, D., Nielsen, I., Sartori, S. Changes in serum, erythrocyte, and urinary magnesium after a single dose of cisplatin combination chemotherapy. Magnesium Res. 1991; 4:123-125.
181. Cappelaere, P., Vincent, A., Staumont, M., Dupuis, B., Gautier, P., Adenis, L. (Continuous Holter cardiac monitoring and chemotherapy by combination with platinum and fluoro-uracil.) Bull. Cancer Paris 1991; 78:261-272.
182. Willcox, J.C., McAllister, E.J., Sangster, G., Kaye, S. Effects of magnesium supplementation in testicular cancer patients receiving cisplatin: a randomized trial. Brit. J. Cancer 1986; 54:19-23.
183. Martin, M., Diaz-Rubio, E., Casado, A., Lopez-Vega, J.M., Sastre, J., Almenarez, J. Intravenous and oral magnesium supplementations in the prophylaxis of cisplatin-induced hypomagnesemia. Results of a controlled trial. Am. J. Clin. Oncol. 1992; 15:348-351.
184. Binet, A., Volfin, P. Effect of an Anti-tumor Platinum complex, Pt (II) Diamino-toluene on mitochondrial membrane properties. Biochim Biophys Acta 1977; 161:182-187.
185. Theophanides, T., Polissiou, M. Magnesium-nucleic acid conformational changes and cancer. Magnesium 5:221-233.
186. Ekimoto, H., Okada, M., Mashiba, H., Shibazaki, C., Takahashi, K. Protection of cisplatin-induced nephrotoxicity by magnesium ion. Magnesium Bull. 12:67, 1990. (from J. Japanese Soc. Mg.Res. 8, 1989).
187. Davey, P., Gozzard, D., Goodall, M., Leyland, M.J. Hypomagnesaemia: an underdiagnosed interaction between gentamicin and cytotoxic chemotherapy for acute non-lymphoblastic leukaemia. J. Antimicrob. Chemother. 1985. 15:623-628.
188. Seelig, M.S. Amphotericin B binding of magnesium: contribution of its toxicity, and therapeutic implications. Magnesium Bull. 1981; 3:80-84.
189. Parsons, F.M., Edwards, G.F., Anderson, C.K., Ahmad, S., Clark, P.B., Hetherington, C., Young, G.A. Regression of malignant tumours in magnesium and potassium depletion induced by diet and haemodialysis. Lancet 1974; 1:243-244.
190. Burman, N.D., Parsons, F.M. Hyperalimentation in the treatment of advanced carcinoma with induced magnesium and potassium depletion. S.A. Med. Tydskrif. 1976; Oct 2:1695-1702.
191. Collery, P., Choisy, H., Millart, H., Pluot, M., Gourdier, B., Simoneau J.P., Charpentier, F., Pechery, C., Coudoux, P. [Is antitumor capability of gallium due to its effect as a competitive inhibitor vis-a-vis magnesium?] Magnesium Bull. 1981; 3:23-25.
192. Manfait, M., Collery, P. ([Role of magnesium and gallium ions on the DNA conformation. In vitro study by Raman spectroscopy.) Magnesium Bull. 1984; 6:153-155.
193. Anghileri, L.J, Robert, J. Radiogallium as a probe for magnesium-binding sites. Magnesium Bull. 1982; 4:197-200.
194. Collery, P., Millart, H., Pluot, M., Anghileri, L.J. Effects of gallium chloride oral administration on transplanted C3HBA mammary adenocarcinoma: Ga, Mg, Ca and Fe concentration and anatomopathological characteristics. Anticancer Res. 1986;1085-1087.
195. Paunier, L., Radde, I.C.: Normal and abnormal magnesium metabolism. Bull. of Hosp. for Sick Childr. (Toronto) 1965; 14:16-23.
196. Paunier, L., Radde, I.C., Kooh, S.W., Conen, P.E.E., Fraser, D. Primary hypomagnesemia with secondary hypocalcemia in an infant. Pediatrics 1968; 41:385-402.
197. Vainsel, M., Vanderville, G., Smulders, J., Vosters, M., Hubain, P., Loeb, H. Tetany due to hypomagnesemia with secondary hypocalcemia. Arch. Dis. Child. 1970; 45:254-258.
198. Seelig, M.S. Prenatal and neonatal mineral deficiencies: magnesium, zinc and chromium. In Clinical Disorders in Pediatric Nutrition Ed. F. Lifshitz, Publ. Marcel Dekker, Inc. New York, 1982; 167-196.
199. Seelig, M.S. Magnesium in pregnancy: special needs for the adolescent mother. J. Am. Coll. Nutr. 1991; 10:566.
200. Duggin, G.G., Dale, N.E., Lyneham, R.C., Evans, R.A., TIller, D.J. Calcium balance in pregnancy. Lancet 1974; 2:926-927.
201. Avioli, L.V. The calcium controversy and the recommended dietary allowance. In The Osteoporotic Syndrome, Detection, Prevention and Treatment., Ed L.V. Avioli, Publ. Grune & Stratton, Inc, Orlando FL, 1987; pp 57-66.
202. Seelig, M.S. Increased need for magnesium with the use of combined oestrogen and calcium for osteoporosis treatment. Magnesium Res. 1990; 3:197-215.
203. Henrotte, J.G.,, Columbani, J., Pineau, M., Dausset, J. Role of H-2 and non H-2 genes in control of blood magnesium levels. Immunogenetics 1984; 19:435-448.
204. Skyberg, D., Stromme, J.H., Nesbakken, R., Harnaes, K. Congenital primary hypomagnesemia, an inborn error of metabolism. Acta Paediat. Scand. 1967; 177:26-27.
205. Stromme, J.H., Nesbakken, R., Normann, T., Skjorten, F., Skyberg, D., Johannessen, B. Familial hypomagnesemia. Biochemical, histological and hereditary aspects studied in two brothers. Acta Paediat. Scand. 1969; 58:433-444.
206. Nordio, S., Donath, A., Macagno, F., Gatti, R. Chronic hypomagnesemia with magnesium dependent hypocalcemia I. A new syndrome with intestinal malabsorption. II. Magnesium, calcium and strontium. Acta paediat. Scand. 1971; 60:441-448, 449-455.
207. Paunier, L. Magnesium malabsorption. Adv. Intern. Med. Pediatr. 1979; 42:113-131.
208. Freeman, R.M., Pearson, E. Hypomagnesemia of unknown etiology. Am. J. Med. 1966; 41:645-656.
209. Gitelman, H.J., Graham, J.B., Welt, L.G. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans. Assoc. Am. Physicians 1966; 79:221-235.
210. Sutherland, L.E., Hartroft, P., Balis, J.U., Bailey, J.D., Lynch, M.J. Bartter's syndrome. A report of four cases, including three in one sibship, with comparative histologic evaluation of the juxtaglomerular apparatuses and glomeruli. Acta Paediat. Scand. 1970; Suppl. 201:1-25.
211. Rodriguez-Soriano, J., Vallo, A., Garcia-Fuentes, M. Hypomagnesaemia of hereditary renal origin. Pediatr. Nephrol. 1987; 1:465-472.
212. Bianchetti, M.G., Girardin, E., Benador-Milsztajn, N., Sizonenko, P.C., Paunier, L. Metabolic studies in primary tubular hypomagnesaemia-hypokalaemia. Magnesium Res. 1988; 1:79-83.
213. Pronicka, E., Gruszczynska, B. Familial hypomagnesaemia with secondary hypocalcaemia--autosomal or X-linked inheritance? J. Inherit. Metab. Dis. 1991; 14:397-399.
214. Henrotte, J.G., Gluckman, E. (Family studies on red blood cell magnesium in leukemia and aplasia.) Magnesium Bull. 1982; 4:93-97.
215. Collery, P., Coudoux, P., Geoffrey, H. Role of magnesium in venous thrombogenesis in cancer: changes in magnesium levels in neoplastic diseases. In Nutrition and Cancer (Proc. 18th Ann Mtg of Am Coll Nutr, 1977), Eds. J. vanEys, M.S. Seelig, B.L. Nichols, Publ. SP Med & Sci Books, 219-231, 1979.
216. Collery, P., Coudoux, P., Geoffrey, H. Magnesium and thrombosis: interrelations with latent tetany, cirrhosis, and cancer. In Nutrition and Heart Disease. (Proc. 19th Ann Mtg of Am Coll Nutr, 1978), Ed H.K. Naito, Publ. SP Med & Sci Books, 325-334, 1982.
217. Darlu, P., Henrotte, J.G. The importance of genetic and constitutional factors in human red blood cell magnesium control. In Magnesium in Health and Disease. (from 2nd Intl Mg Sympos, Montreal, Canada, 1976), Eds. M. Cantin and M.S. Seelig, Publ. Spectrum Press, NY, 1980; 921-927.
218. Henrotte, J.G. (Genetic factors of magnesium metabolism regulation in man.) Magnesium Bull. 1981; 3(1a):237-248.
219. Feray, J.C., Franck, G., Garay, R., Henrotte, J.G. Inter-individual differences in red cell Mg2+ contents are related to the activity of a Na+:Mg2+ exchanger; possible relationship with HLA-associated genetic factors. Magnesium Res. 1989; 2:124.


No comments:
Post a Comment